Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Neuroscience
Silicon Nitride Microtubes Direct Neuron Growth
Bioengineering: Arrays of silicon nitride microtubes could be used to design synthetic neural circuits for studying the brain and developing new medical devices
by Katherine Bourzac
November 6, 2014

Bioengineers have connected electronics to neurons in devices such as cochlear implants and even experimental brain-computer interfaces that make it possible to control a robotic arm with thought. More sophisticated devices that integrate neurons with electronics might lead to prosthetic limbs with a seemingly natural sense of touch, and synthetic neural circuitry that scientists could use to study how the brain processes information or what goes wrong in neurodegenerative diseases.

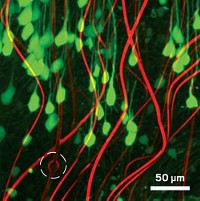
As a step toward these applications, a team of researchers has developed a way to direct the connection-forming arms of neurons. They use silicon nitride microtubes on glass slides to encourage the cells’ axons to grow in specific directions (ACS Nano 2014, DOI: 10.1021/nn504876y).
To control the growth of neurons in the lab, scientists previously have patterned cell-adhesive factors on the surfaces of culture dishes and hoped that neurons followed them. This method is useful but has its limitations, says William Shain, a neurobiologist at the University of Washington Medical Center, who was not involved in the new work. “These techniques are two-dimensional, but cells normally grow in three-dimensional space,” he says.
Justin C. Williams of the University of Wisconsin, Madison, had tried mimicking this 3-D environment by growing nerve cells inside the narrow channels of microfluidic devices. But microfluidics are typically made out of rubbery materials that aren’t compatible with making the electronics needed to sense and stimulate these cells. This led him to experiment with growing nerve cells in rolled-up tubes of inorganic electronic materials such as silicon germanium. Unfortunately, the tubes were too wide to provide an effective growing environment for single neurons, and they didn’t let light in—blocking the view of microscopes.
So he started collaborating with Xiuling Li, an electrical engineer at the University of Illinois, Urbana-Champaign, who makes arrays of transparent silicon nitride tubes ranging in size from a few nanometers to hundreds of micrometers in diameter. The researchers thought tubes slightly larger than the diameter of a typical axon would create a cozy environment to encourage neurons to grow, one that’s analogous to the 3-D environments they naturally find in brain and nerve tissue.
To build the tubes, Li’s group starts by growing two planar layers of silicon nitride. The bottom layer is grown with strain that makes the material want to expand, Li says; the top film has strain that makes it want to contract. When they etch through the films, the strain is released, and the material rolls up into single tubes or double scroll structures (Nanotechnology 2013, DOI: 10.1088/0957-4484/24/47/475301).
Li’s group made arrays of tubes 4.4 µm in diameter and 50 µm long on the surface of glass slides. When the team cultured cortical neurons on the slides, they observed that the cells’ axons grew aimlessly until they found a microtube. The axon then entered the tube, and its growth accelerated 20-fold. The cell body remains outside while the axon snakes through.
The observations demonstrate that these tubes could potentially be used to design networks of neurons in culture, the researchers say. Williams thinks that the growth acceleration occurs because the cells have more surface area to grab onto, compared with the flat surface of the glass slide. Also, silicon nitride has an intrinsic surface charge that may provide a stimulating cue to the cells, he says.
Williams and Li are now working on adding chemical sensors and electrical components inside the tubes, possibly allowing scientists to make measurements of the cell’s activity at multiple spots along a single axon. They’re also working on growing arrays of tubes to create complex neuronal networks similar to the 3-D structures found in the brain.
The UW Medical Center’s Shain sees a potential therapeutic application for the tubes: helping axons grow across long distances to repair injuries. In the body, neurons typically won’t grow past a scar. With further development, these microtubes could be used to rebuild severed nerve bundles, he says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter