Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Eye Exam For Chaperone
Molecular Biology: NMR study of protein fragment reveals sequential binding mechanism
by Stu Borman
January 5, 2015
| A version of this story appeared in
Volume 93, Issue 1

A new study reveals the likely mechanism that a protein called human α-crystallin uses to protect the lens of the eye from clouding or becoming opaque. The work could lead to new drugs to prevent cataract formation.
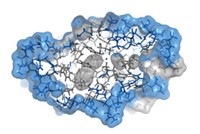
The human eye lens contains a protein called γD-crystallin that can unfold, oxidize, and aggregate to form cataracts. Human α-crystallin protects the lens by inhibiting γD-crystallin aggregation.
But how this chaperone protein works on a molecular level has been a mystery. Because human α-crystallin has a variable number of subunits, it has been difficult to use techniques such as nuclear magnetic resonance (NMR) spectroscopy to study its shape changes and binding interactions as it performs its protective role.
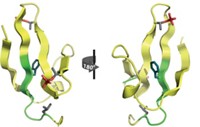
In 2000, ophthalmology professor Krishna Sharma of the University of Missouri and coworkers discovered that mini-α-crystallin (MAC), a tiny 19-residue fragment of α-crystallin, has chaperone properties similar to those of the parent protein.
Recognizing that MAC is much easier to study than the parent protein, researchers have now probed it with NMR to see how MAC, and presumably α-crystallin as well, works its protective magic. The study was carried out by chemistry professors Jayanti Pande and Alexander Shekhtman and coworkers at the University at Albany (Biochemistry 2014, DOI: 10.1021/bi5014479).
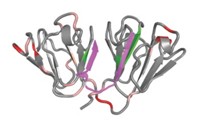
The study suggests that MAC prevents cataracts by binding weakly to the exterior of natively folded γD-crystallin and then moving to the interior when heat or chemical stress causes γD-crystallin to unfold.
“The lower-affinity binding to sites on the native state suggests a two-state recognition process, which would be kinetically very efficient, in which a weakly bound chaperone scans the protein for unfolded regions,” comments Jonathan King, a crystallin and protein misfolding specialist at MIT. “The authors’ NMR data directly identify interactions with buried core residues presumably exposed during unfolding.”
Because human α-crystallin likely shares this mechanism, at least to some extent, the work gives a first molecular view of the mechanism of action of the lens-protective parent protein. The findings could thus aid the design of MAC mimics as anticataract drug candidates. “For example, you could make a


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter