Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Amyloid Fibril Has Unusual Structure
Structural Biology: Researchers generate Alzheimer’s-related fibril, find it has S-shaped configuration
by Celia Henry Arnaud
May 14, 2015
| A version of this story appeared in
Volume 93, Issue 20

Many scientists believe that plaques made of amyloid peptide contribute to brain degeneration in people with Alzheimer’s disease. Moreover, fibrils of a 42-amino-acid fragment of this peptide are increasingly thought to be the main culprits in triggering plaque formation. But structural information about these fibrils has been hard to come by because they are heterogeneous.
Yoshitaka Ishii of the University of Illinois, Chicago, and coworkers now report generating a homogeneous Aβ-42 fibril in vitro and obtaining a model of its structure with solid-state nuclear magnetic resonance spectroscopy (Nat. Struct. Mol. Biol. 2015, DOI: 10.1038/nsmb.2991). The structure differs significantly from that of Aβ-40, Aβ-42’s shorter and more abundant sibling, which has also been implicated in Alzheimer’s.
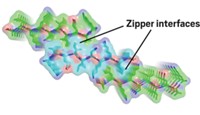
Previously reported Aβ-40 fibrils are U-shaped with parallel β-sheets connected by a flexible loop. In contrast, this particular Aβ-42 fibril contains three connected β-sheets that adopt an S-shaped configuration.
Different interactions stabilize the Aβ-40 and Aβ-42 structures. The shorter peptide is held together by a salt bridge between lysine 28 and aspartic acid 23. Instead of that interaction, Aβ-42 forms a salt bridge between lysine 28 and alanine 42, a residue that doesn’t exist on the shorter peptide.
Previous structural studies by Robert Tycko at the National Institutes of Health and others have shown that amyloid-β fibrils having a given sequence can adopt multiple structures with subtle differences in configuration.
The new work is important, Tycko says, “because it demonstrates that conformational variations in amyloid-β fibrils can be dramatic.” In addition, the study suggests an explanation for why fibrils formed by Aβ-40 and Aβ-42 don’t “cross-seed,” or trigger one another to form fibrils, he says. “It remains to be seen whether the fibril structure described in this paper exists in human brain tissue,” he notes.
If the structure is found in the brain, drugs that have already been designed to optimally obstruct an arched β-motif in Aβ-40 may not work as well against Alzheimer’s, which might be caused by the more toxic Aβ-42 fibrils, Ishii and coworkers say.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter