Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Flexible Electronics Injected Into Mouse Brains
Bioelectronics: Researchers use syringes to insert submicron-thick mesh electronics to record neuron activity in living animals
by Michael Torrice
June 10, 2015
| A version of this story appeared in
Volume 93, Issue 24

Syringes deliver drugs, vaccines, and cells deep into the tissues of animals and people. Now a study adds electronic devices to that list.
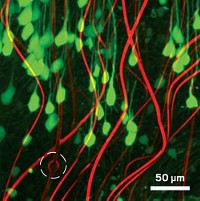
A team of researchers report extremely flexible, centimeter-wide mesh electronics that they can inject into the brains of mice, allowing them to record neuron activity without damaging surrounding tissue. These devices could lead to improved neural implants that interact with brain tissue to treat diseases such as Parkinson’s or to help neuroscientists study brain circuitry.
The new devices “will likely change how we think about the possibilities when merging electronics with tissue” and could lead to interesting new technologies, says Michael R. Bruchas, a neurobiologist at Washington University in St. Louis who was not involved in the work.
Brain implants are already approved by the Food & Drug Administration to treat some forms of Parkinson’s disease and other neurological illnesses.
Despite the success of these implants, they all have a significant limitation, says Charles M. Lieber of Harvard University. Their mechanical properties, such as flexibility, do not match those of the brain tissue surrounding them. So when the brain moves in the skull, the devices don’t respond the same way the tissue does. This leads to the implant rubbing against the tissue, causing cell damage that elicits an immune response. These reactions can build up scar tissue around the implant, affecting device performance.
The new electronic devices, developed by Lieber and colleagues, are 10,000 to 1 million times as flexible as previously reported implantable devices. A month after the researchers injected the new devices into mouse brains, they saw little to no sign of a reaction. For instance, neuron density around the electronics remained unchanged (Nat. Nanotechnol. 2015, DOI: 10.1038/nnano.2015.115).
To achieve this extreme flexibility, the team made the electronics significantly thinner than previous devices—0.8 µm versus more than 10 µm. The mesh structure, which Lieber describes as a version of chicken wire, is also less stiff than the continuous sheets of polymer used in other devices.
The new implants consist of thin gold wires encapsulated by a biocompatible polymer. These wires intersect across the mesh, forming a series of tiled parallelograms. This design allows the devices to roll up when sucked into a syringe and then easily flow out the needle when injected into tissue. In fact, the team could inject mesh electronics that were more than 30 times as wide as the internal diameter of a needle without clogging.
The ability to inject the electronics, instead of implanting them via surgery, allows “for reaching deeper brain structures or harder-to-reach places than more traditional electronic implants can,” Bruchas says. “It could allow for integration or sensing of brain regions that have been previously difficult to interact with.”
Lieber says his team is now testing the biocompatibility of the mesh for periods longer than a month. The researchers also plan to investigate applications such as recording activity from single neurons in the retina of mice and co-injecting the mesh with stem cells to see whether they can regenerate tissue in mice that have had strokes.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter