Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Nickel Shines In Ammonia Couplings
Chemists use nickel to replace palladium in an important catalytic reaction for making aryl amines
by Stephen K. Ritter
January 19, 2015
| A version of this story appeared in
Volume 93, Issue 3
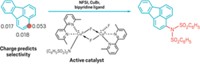
By developing a stable nickel catalyst system, a Canadian research team has fulfilled a quest to move beyond using expensive palladium catalysts in cross-coupling reactions to make aryl amines from ammonia. Ammonia is the simplest and most abundant N–H source in chemistry, with virtually all synthetic nitrogen-containing compounds originating from the inexpensive feedstock. Industrial syntheses of amines from ammonia, however, typically require heterogeneous catalysts at relatively high temperatures and pressures, which results in modest product selectivity. Only recently have chemists devised homogeneous palladium catalysts for the task, which allow for more selective ammonia couplings under milder conditions. The approach taken by Andrey Borzenko, Mark Stradiotto, and coworkers of Dalhousie University, in Halifax, Nova Scotia, is the first example of nickel-catalyzed arylation of ammonia to make amines. The researchers used air-stable Ni(cyclooctadiene)2 or NiCl2(dimethoxyethane) with a ferrocenyl phosphine ligand known as JosiPhos to link up a range of substituted aryl and heteroaryl bromides, chlorides, and tosylates with ammonia (one shown) to make diverse aryl and heteroaryl amines (Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201410875).



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter