Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Cheap, Quantitative Synthesis of Quantum Dots
ACS Meeting News: Library of thiourea compounds lets chemists dial in properties of colloidal nanocrystals
by Bethany Halford
August 24, 2015
| A version of this story appeared in
Volume 93, Issue 33
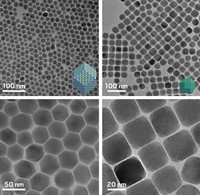
Few nanomaterials possess the flashiness of quantum dots. These colloidal nanocrystals glow in a rainbow of colors determined by their size, leading researchers to eye them for several technologies, including displays and solid-state lighting. The materials have yet to achieve their full promise because making them can be expensive and can generate a lot of waste. For example, metal sulfide quantum dots are usually made by adding a sulfide source to a metal salt solution and then stopping the reaction when the nanocrystals reach the desired size. This process can lead to variable yields and crystal sizes, as well as wasted starting material. Jonathan S. Owen and chemists at Columbia University have developed a library of inexpensive sulfide sources that produce quantum dots in quantitative yield (Science 2015, DOI: 10.1126/science.aaa2951). The library consists of thousands of thiourea compounds. By varying the thioureas’ substituents, the researchers tuned the rate at which the compounds produce sulfide, therefore giving the researchers control over nanoparticle size. Owen’s team also developed a similar synthetic strategy for making selenide quantum dots as well as nanocrystals with a metal-selenide core surrounded by a metal sulfide shell.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter