Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
A Little Palladium Does A Lot Of Cross-Coupling
Green Catalysis: Iron nanoparticles containing traces of palladium facilitate workhorse reactions in a recyclable aqueous solution
by Stephen K. Ritter
September 7, 2015
| A version of this story appeared in
Volume 93, Issue 35
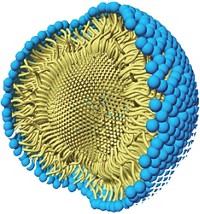
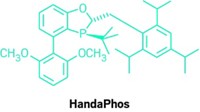
As part of their efforts in developing greener chemistry, Bruce H. Lipshutz and his group at the University of California, Santa Barbara, have already pared Suzuki-Miyaura cross-coupling reactions down to no organic solvent, little if any heating, and a minimum of palladium catalyst. But the fact that their recyclable aqueous reaction system still requires a relatively expensive amount of scarce palladium has bothered Lipshutz. His group has now found a way to reduce the amount of palladium further. Working in collaboration with Novartis, the researchers discovered that some types of inexpensive, commercially available FeCl3 naturally contain parts-per-million amounts of the precious metal. When the team processes the iron salt into nanoparticles, there’s just enough palladium on board to catalyze cross-couplings (Science 2015, DOI: 10.1126/science.aac6936). The chemistry is made possible by a designer surfactant Lipshutz invented, called TPGS-750-M, that when added to water forms lipophilic nanomicelles. The size and structure of these tiny reaction vessels control the flux of reactants, catalyst, and product into and out of the nanomicelles to optimize reaction rates. The researchers show that the new iron nanoparticles contain about one-hundredth the amount of palladium typically used in cross-coupling reactions and that it’s free with the purchase of FeCl3.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter