Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Making Glycoconjugate Vaccines More Uniform And Effective
Immunology: Study demonstrates that vaccine efficacy depends on amino acid site where researchers attach sugar antigens to carrier protein
by Stu Borman
September 17, 2015
| A version of this story appeared in
Volume 93, Issue 37

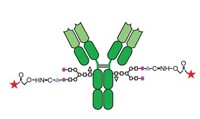
Glycoconjugate vaccines can spur the body’s immune system to seek out and attack pathogens or cancer cells on the basis of which specific sugar chains decorate those targets. Approved vaccines for conditions such as meningitis and pneumonia work through such sugar-based antigens.
To make glycoconjugate vaccines, scientists nearly always attach the sugars randomly to amino acids in carrier proteins to enhance the carbohydrates’ ability to elicit an immune response.
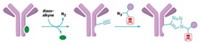
Now, researchers have used several reactions to link sugars to specific amino acids in a carrier protein and have found that where they attached the carbohydrates affected the level of responses to the vaccine (Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201506112).
The work shows that better control over attachment points used in the conjugation process could aid the development of better characterized vaccines with improved efficacy and more consistent molecular properties.
The investigators—Qi-Ying Hu of Novartis Institutes for BioMedical Research, in Cambridge, Mass.; Francesca Micoli of Sclavo Behring Vaccines Institute for Global Health, in Siena, Italy; and coworkers—note that this is the first comparative study of the immune effects of different sugar conjugation sites.
The sugar, or glycan, in the study is a lipopolysaccharide antigen from Salmonella enterica serovar Typhimurium, which causes a form of food poisoning that can be fatal, especially in immunocompromised patients. There are no current vaccines for this pathogen.
The research team used several site-selective conjugation methods, including two they developed, to attach the antigen via a linker to specific cysteines, lysines, tyrosines, glutamates, and aspartates in a carrier protein called CRM197. When they injected the vaccines into mice, the amount of antibacterial antibodies generated varied significantly depending on the conjugation site—as much as an order of magnitude.
Attaching a single sugar to both cysteines of a disulfide bond produced one of the more productive glycoconjugates. The conjugate was as active as ones with multiple sugars. This demonstrates how picking a specific sugar-linkage site can help optimize glycoconjugate potency, Micoli says, because a conjugate with multiple sugars is often more effective than a conjugate with just one.
Gonçalo Bernardes, an expert on site-selective protein modification at the University of Cambridge and the University of Lisbon, comments that this is the first structure-activity relationship study that clearly shows that attachment site can play a key role in immune responses to glycoconjugates. Conjugation site should be taken into consideration in the design of future carbohydrate-based vaccines, he says. However, Bernardes notes that scientists will also need to assess the effects on vaccine efficacy of the lengths of chemical linkers used to attach carbohydrate antigens to amino acids.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter