Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Vitamin B-12 Expands Its Regulatory Role
Bioinorganic Chemistry: Structural study unveils how adenosylcobalamin helps guide light-dependent gene regulation
by Stephen K. Ritter
October 5, 2015
| A version of this story appeared in
Volume 93, Issue 39
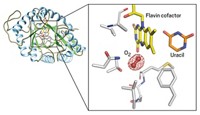
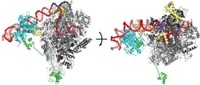
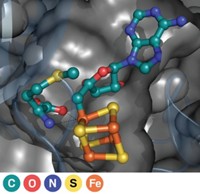
Adenosylcobalamin, one of the active forms of vitamin B-12, is known for its job as a cofactor in radical-based enzyme reactions and as a modulator of gene expression via mRNA riboswitches. Scientists have more recently discovered that many bacteria also use the molecule as a light-capturing chromophore for photoreceptor proteins that help protect cells from sunlight-induced DNA damage. Researchers are now reporting the mechanistic details behind this new process (Nature 2015, DOI: 10.1038/nature14950). Marco Jost and Catherine L. Drennan of MIT, along with S. Padmanabhan of the Institute of Physical Chemistry Rocasolano, in Madrid, and Montserrat Elías-Arnanz of the University of Murcia, in Spain, and coworkers obtained X-ray crystal structures of one photoreceptor protein, the transcription factor CarH, in three different states—in the dark, bound to DNA, and after being exposed to light. The structural snapshots reveal how, in the dark, adenosylcobalamin guides formation of a CarH tetramer that binds DNA to prevent transcription. When light hits the bacteria, it photolyzes adenosylcobalamin and induces a conformational change in CarH that causes the protein complex to dissociate from DNA, which triggers the bacteria to express genes that produce carotenoid molecules to counter light-induced oxidative damage. These mechanistic details could help scientists develop light-directed control of gene transcription or methods to control interactions between proteins.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter