Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
How Chemistry Is Helping Physicists Detect Neutrinos
Researchers formulate new liquids to reveal properties of elementary particles
by Jyllian Kemsley
October 27, 2015
| A version of this story appeared in
Volume 93, Issue 43
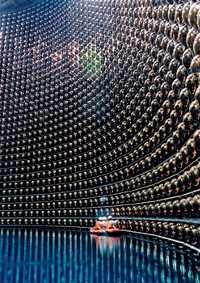
To learn that neutrinos have mass, this year’s Nobel Prize in Physics recipients, Takaaki Kajita and Arthur B. McDonald, had to first detect the subatomic particles in huge, liquid-filled underground tanks. The rock above these subterranean facilities filters out most cosmic rays bombarding Earth, allowing scientists to observe small, chargeless neutrinos.

During the experiments for which they were honored, Kajita and McDonald merely used water as a detection medium at Japan’s Super-Kamiokande and Canada’s Sudbury Neutrino Observatory (SNO), respectively. Going forward, however, neutrino-hunting physicists plan to use chemistry to make more complex fluids with increased sensitivity to detect neutrinos from different sources and reveal new properties of the particles.
Water-based neutrino detection is based on Cherenkov radiation, which is produced when charged particles move through water faster than light. When a neutrino collides with a water molecule, charged particles are ejected. Just as a plane traveling faster than the speed of sound creates a sonic boom, the charged particles give off an optical shock wave that can be detected by photomultiplier tubes lining a facility’s tank. Physicists used this detection strategy with normal water at Super-Kamiokande and with D2O at SNO; the IceCube observatory at the South Pole is currently taking advantage of frozen water.
But there’s another detector approach available to neutrino hunters: scintillation. In this scheme, a neutrino interacts with the protons and neutrons of an organic liquid, and the reaction produces a flash of light. A fluorescent additive shifts the wavelength of emitted light to a detectable region. For instance, at Japan’s Kamioka Observatory, the liquid is composed of dodecane, 1,2,4-trimethylbenzene, and the fluorophore 2,5-diphenyloxazole. At China’s Daya Bay project, the liquid is linear alkylbenzenes doped with gadolinium.
This gadolinium adds another layer of sophistication to neutrino detection. When a neutrino’s antimatter counterpart, an antineutrino, collides with a proton, the interaction produces a positron and a neutron. The positron produces one light flash. Gadolinium can absorb the neutron and emit gamma radiation, yielding a second flash that further confirms the antineutrino detection.
Now, chemists are looking at ways to create water-based scintillation liquids that combine all the different neutrino detection mechanisms to expand the range of neutrinos that can be studied. For example, SNO detected neutrinos produced by the sun. The liquid scintillator planned for Canada’s next-generation facility, SNO+, will also detect lower-energy antineutrinos produced by radioactivity deep inside Earth, says Mark C. Chen, SNO+ project director and physics professor at Queen’s University in Ontario.
The big challenge with combination liquids is making the water and the organic components—linear alkylbenzenes are still a favorite—miscible and stable for at least a decade, says Minfang Yeh, who leads the neutrino and nuclear chemistry group at Brookhaven National Laboratory. To help hold the solution together, his group has experimented with various surfactants, including polyethylene glycol octylphenol ether—commonly known as Triton X-100—and linear alkylbenzene sulfonate derivatives. All components also need to be compatible with whatever material contains the liquid. For example, SNO+ has an acrylic spherical tank.
The liquid scintillator at SNO+ will also incorporate 2,5-diphenyloxazole as well as tellurium. SNO+ scientists hope to use the tellurium to detect an extremely rare process called neutrinoless double beta decay that occurs when nuclei decay and emit two neutrinos—but the neutrinos annihilate each other during the decay process. “That’s never before been observed,” Chen says. “If we can measure it, it will tell us that neutrinos are their own antiparticle, which will also help us understand why neutrinos are so lightweight.” In this scenario, the particles currently identified as antineutrinos spin in the opposite direction as neutrinos, resulting in different reactions with protons and neutrons.
To get the tellurium in solution, Yeh’s group at Brookhaven is collaborating with researchers at Queen’s University and the University of Oxford to experiment with surfactants. The researchers are also reacting tellurium with different complexing ligands, such as carboxylates or diols, to generate an organotellurium complex that will be stable in a new and improved scintillation liquid.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter