Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Amides Succumb To Suzuki–Miyaura Coupling, Thanks To Nickel Catalyst
Organic Chemistry: Abundant metal opens new avenues in popular reaction pathway
by Bethany Halford
November 16, 2015
| A version of this story appeared in
Volume 93, Issue 45
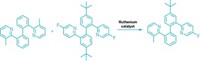
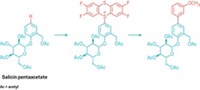
The first nickel-catalyzed Suzuki-Miyaura coupling using an amide derivative as a cross-coupling partner has been reported by chemists at UCLA (Nat. Chem. 2015, DOI: 10.1038/nchem.2388). Suzuki-Miyaura couplings are wildly popular for forging C–C bonds: The prototype reaction uses a palladium catalyst to link an organoboron reagent with an electrophile, such as an organohalide. In recent years, chemists have investigated nickel catalysts for these types of reactions because the metal is more abundant and less expensive. Neil K. Garg, Nicholas A. Weires, and Emma L. Baker found they could use a nickel catalyst to activate an amide’s C–N bond so that it undergoes cross-coupling with a boronic ester to produce an acyl C–C bond. Organic chemists have historically considered amides to be inert substrates, the authors note, but this work adds to a growing body of evidence that certain conditions can make them useful in organic synthesis. The new reaction tolerates a broad range of functional groups, including ketones and amines. The UCLA chemists used the transformation to create an antiproliferative agent (shown) on a gram scale.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter