Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
New Desalination Method Shocks The Salts Out Of Water
Environment: Prototype device uses electrodialysis to produce freshwater
by Elizabeth K. Wilson
November 23, 2015
| A version of this story appeared in
Volume 93, Issue 47

A novel method for water desalination separates salt water into briny and fresh streams with the help of an electric shock wave (Env. Sci. & Tech. Lett. 2015, DOI: 10.1021/acs.estlett.5b00303)
As more and more people live in areas affected by drought or contaminated water, desalination is becoming an important way to meet global drinking water needs. So scientists continue to develop ever simpler and less expensive desalination methods.
Current technologies, for example, frequently rely on membranes to filter out ions. These membranes eventually get clogged and must be replaced, increasing costs.
In recent years, scientists have been exploring electrochemical methods of removing salts from water. For example, one group has designed a method where pairs of capacitive electrodes are first dipped in salt water to absorb salt, and then in a brine chamber, where they discharge the salt.
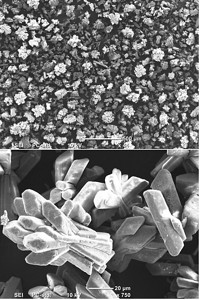
Now, a team led by Martin Z. Bazant at Massachusetts Institute of Technology has demonstrated another method with a prototype device composed of porous glass beads, known as a frit, sandwiched between two electrodes. They applied a current across the electrodes as salt water flowed through the frit, causing some areas of the stream to collect more ions while others became ion depleted.
When the electrical current became strong enough, it induced a shockwave that separated these regions into two streams—one briny, one fresh—a phenomenon known as shock electrodialysis.
The device continuously removed up to 99.99% of salts, including sodium, chloride and other ions, from a saltwater stream.
“This new system seems very promising indeed,” says P. Maarten Biesheuvel, a principal scientist at Wetsus, European Centre of Excellence for Sustainable Water Technology, who helped develop the capacitive electrode desalination technology.
Biesheuvel says that compared with his method, the electroshock method “has the major advantage that we don’t need any moving parts,” he says.
The method may have other advantages, says the new study’s coauthor Sven Schlumpberger. For example, the electoral current might kill pathogenic bacteria.
The MIT team says the next step will be to design scaled-up versions of their prototype to test the method’s industrial feasibility.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter