Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Dialing Direct For Hydrogen Peroxide
Catalysis: Study reveals a mechanism for the direct reaction between hydrogen and oxygen on palladium nanoclusters
by Mitch Jacoby
December 7, 2015
| A version of this story appeared in
Volume 93, Issue 48
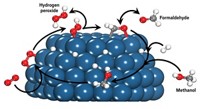
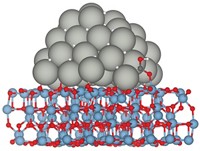
Synthesizing hydrogen peroxide directly from hydrogen and oxygen would lower the cost of H2O2 production, making the oxidizing reagent an environmentally friendly substitute for chlorinated oxidizers in organic chemistry, wastewater treatment, and other processes. Direct synthesis involving metal catalysts generates significant amounts of water, which would necessitate costly separation and concentration steps. Instead, H2O2 is typically made from anthraquinones via a multistep process. But the catalytic inefficiencies could be overcome if the reaction mechanism were understood in fine detail, according to chemical engineers Neil M. Wilson and David W. Flaherty of the University of Illinois, Urbana-Champaign. To probe the mechanism, the team used nanosized palladium catalysts to investigate H2O2 synthesis over a range of hydrogen and oxygen pressures and solvent pH values. Their findings indicate that, in contrast to the commonly accepted mechanism, the reaction proceeds as follows: O2 adsorbs on the catalyst, draws two electrons from the catalyst, and reacts with two hydrogen ions from solution to form H2O2. Elsewhere on the catalyst surface, H2 adsorbs and dissociates, supplying the needed electrons and hydrogen ions (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b10669).


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter