Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Functionalized Biaryls By Organocatalysis
Organic Synthesis: Organic acid-catalyzed reaction yields compounds that are promising as ligands and catalysts
by Bethany Halford
December 7, 2015
| A version of this story appeared in
Volume 93, Issue 48
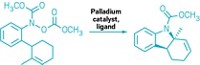
Biaryl compounds with a 1,1´ linkage are popular as ligands and catalysts for preparing chiral drug molecules. The 1,1´-biaryl structure also shows up in numerous natural products. But making this chemical motif isn’t trivial, particularly when the biaryls aren’t symmetrical. Syntheses usually require prefunctionalizaton or transition-metal catalysts. Chemists led by Rice University’s László Kürti have now developed an organocatalytic approach to making nonsymmetrical functionalized 1,1´-biaryls (Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201508419). The reaction weds a quinone monoacetal to a hydroxyaromatic compound with the help of an organic acid catalyst (example shown). The one-step reaction requires no metals and uses inexpensive starting materials. Kürti’s team reports it was able to scale up one example of the reaction to produce 26 g of the biaryl compound—a feat the chemists say shows the reaction is well-suited to syntheses of ligands and catalysts. The researchers believe the reaction’s mechanism most likely involves formation of a mixed acetal followed by a sigmatropic rearrangement. With this reaction chemists will be able to make many different kinds of nonsymmetrical functionalized 1,1´-biaryls, which they can use as catalysts or ligands for known or new reactions, Kürti tells C&EN.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter