Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
A Liquid With Holes In It
Supramolecular Chemistry: Dissolved cage compounds created a fluid with permanent porosity
by Stu Borman
December 21, 2015
| A version of this story appeared in
Volume 93, Issue 49

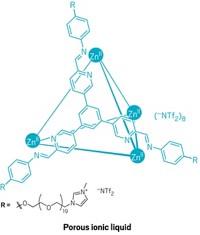
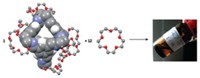
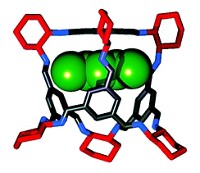
A new type of material developed this year, a liquid with permanent porosity, combines the merits of porous solids and continuous fluids. The systems developed by Stuart James of Queen’s University Belfast and coworkers could be used to separate mixtures of gases by size and to catalyze chemical processes, if the holey liquids can be made at a reasonable cost (Nature 2015, DOI: 10.1038/nature16072). Porous solids such as zeolites have proved useful for separating molecules and as catalysts. But being solids limits their applications. They can’t readily flow through channels or be smoothed onto surfaces. Liquids flow, but they aren’t usually holey. They do have intermolecular spaces, but those spaces are tiny, and bubbles blown into liquids to instill porosity generally dissipate quickly. The new holey liquids are made from hollow organic cage molecules built by coupling 1,3,5-triformylbenzene with one or two types of diamines and dissolving the cage molecules in a solvent. The cage openings are too small for the solvent molecules to enter and block, ensuring the liquids’ porosity. In their first effort, the researchers made a cage from triformylbenzene and a crown ether-functionalized diamine. The diamine decorated the cage surface with crown ether groups to make it soluble in a crown ether solvent. This initial holey liquid was viscous and hard to synthesize. So collaborators Andrew I. Cooper and Rebecca L. Greenaway at the University of Liverpool made a second version from triformylbenzene and two simple alkyldiamines in hexachloropropene solvent. The resulting liquid was less viscous and easier to make. Sheng Dai of Oak Ridge National Laboratory, who earlier helped design nanoporous liquids made up of surface-functionalized hollow colloidal silica nanoparticles, commented that the James group’s holey liquids “will open up new frontiers in how we think about porosity.”
C&EN's YEAR IN REVIEW
Top Headlines of 2015
- Chemical Makers Looked To Big Deals
- NASA Got Up Close And Personal With Pluto
- Opposition To Neonicotinoids Intensified
- 2015 Nobel Prizes In Science At A Glance
- Climate Pact Clinched
- Little Good News For Chemistry Job Outlook In 2015
- Pfizer To Merge Again, This Time With Allergan
- Oil And Gas Industry Under Pressure
- Greening Up Fracking
- An Industry In Spin Cycle
- Finally, Emoji For Chemists
- A Big Deal For Chemists
- Tianjin Explosion Put Spotlight On Safety
- Women Assumed Leadership Roles At American Chemical Society
- Artificial Ingredients In The Crosshairs
- Jacqueline K. Barton, Unwavering Chemistry Champion
- House Science Committee Chair Pummeled Science Agencies
- NIST Veteran Became U.S. Government's Top Chemist
- Gene-Editing Technique Raised Ethics Questions
- World Chemical Production At A Glance
- Classroom Fires During Science Demonstrations Spark Concern
- Climate Was Right For Deal-Making
- American Chemical Society Dives Deeper Into Open Access With The Debut Of ACS Omega
- Lego Began Research On Switching To A Biobased Plastic
- New Chair Took The Helm At Troubled Chemical Safety Board
- Genetically Modified Foods In The Spotlight
- Overhaul Of U.S. Chemical Law Moved
- ACS Scholars Program Turned 20
- American Chemical Society Expanded Its Global Reach
- Scientists Called For Standardized Antibodies
Top Research of 2015
- Flexible Electronics You Can Inject
- Special Delivery For Sensitive Reagents
- Yeast Programmed For Opioid Total Synthesis
- Miracle Machine Builds Molecules On Demand
- Nickel Shines As A Catalyst
- 3-D Printing Takes On A New Dimension
- Atomically Thin Films Grow In Number
- Digging In The Dirt Yields Novel Bacteria Fighter
- Keeping GMOs On A Leash
- A Liquid With Holes In It
- Electron Microscopy Provides Unprecedented Close-ups
Revisiting Research of 2005


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter