Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Protein Arginine Deiminases Come Into Focus
Chemical Biology: Scientists home in on drug targets for cancer, inflammation
by Bethany Halford
January 28, 2015

A clearer picture is emerging of the novel drug targets known as protein arginine deiminases, or PADs, and how to block them, thanks to two recent reports. The findings could have implications for treating autoimmune disorders, cancer, and inflammatory diseases.
PAD enzymes mediate the conversion of arginine residues to the α-amino acid citrulline. The citrullination process is known to be important in the formation of structural tissues such as skin, hair, and the myelin sheath that insulates nerves. But sometimes PADs can be overactive, creating an excess of the citrullinated proteins that have been implicated in several diseases.
For example, patients with rheumatoid arthritis make antibodies to citrullinated proteins. “These antibodies turn out to be the most specific diagnostic for the disease,” says Paul R. Thompson, a biochemistry professor at the University of Massachusetts Medical School, in Worcester, who studies PADs. He notes that it’s possible to detect these antibodies in blood up to 10 years before a patient develops clinical symptoms of rheumatoid arthritis. “It looks like people are sick before they know they are sick,” he explains.
By making an inhibitor that blocks one of the PAD enzymes associated with human disease, such as PAD2 or PAD4, scientists might be able to disrupt the formation of certain citrullinated proteins and possibly prevent disease.
To that end, Thompson and a team of researchers solved 27 crystal structures of PAD2 to try to understand how the enzyme works and how six calcium ions associated with the enzyme play a role in its function (ACS Chem. Bio. 2015, DOI: 10.1021/cb500933j). The researchers found that three of the six calcium-binding sites act as a switch for PAD2, by triggering a conformational change that facilitates the binding of the sixth calcium ion. By designing a compound that inhibits calcium binding to the swtich, Thompson explains, it might be possible to shut the enzyme down. He has cofounded a biotech firm—Padlock Therapeutics—with the aim of developing drugs that do just that.
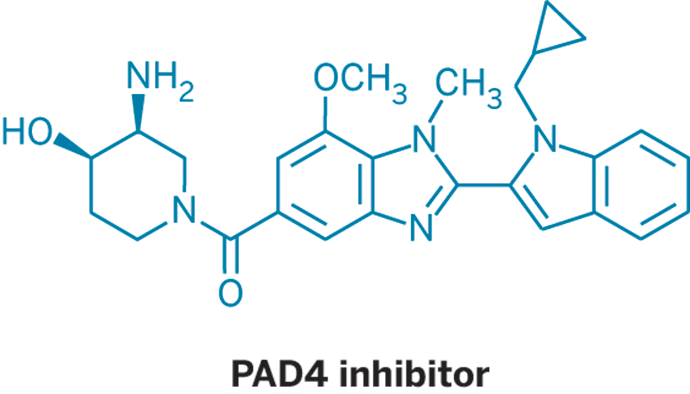
In a related paper, on which Thompson collaborated, researchers led by Huw D. Lewis of GlaxoSmithKline report small molecules capable of selectively and reversibly inhibiting PAD4 (Nat. Chem. Bio. 2015, DOI: 10.1038/nchembio.1735). They also note that these molecules inhibit the formation of the pathogen-fighting fibers known as neutrophil extracellular traps, or NETs. Aberrant NETs have been linked to diseases such as lupus and vasculitis.
“This is the first definitive proof that PAD4 is a very good target for these diseases,” Lewis tells C&EN.
Patrick Venables, an expert in citrullination and rheumatoid arthritis at the University of Oxford, says the two papers “represent an important progression from studies of the pathology of citrullination into the potential for development of a completely novel class of drugs for targeting chronic inflammation and cancer.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter