Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Shining A Brighter Light On Protein Interactions
Analytical Methods: Promega reports a supersensitive approach for studying protein-protein interactions inside living cells
by Erika Gebel Berg
June 4, 2015
Interactions between pairs of proteins inside cells are fundamental to cellular operations, so when things go awry in the cell in diseases, protein-protein interactions are sometimes to blame. To test drug candidates that might target these interactions, researchers would like to be able to quantify such interactions in living cells, where they matter most. Now, researchers at the life sciences company Promega have developed a method that does just that, using a very bright luminescent reporter protein system. The method is sensitive enough, they say, to detect protein interactions within a single cell (ACS Chem. Biol., 2015, DOI: 10.1021/acschembio.5b00143).

More than 15 years ago, researchers developed a method known as bioluminescence resonance energy transfer (BRET) to spy on intracellular protein interactions (Proc. Nat. Acad. Sci. USA 1999, DOI: 10.1073/pnas.96.1.151). BRET involves inserting a pair of plasmids—small circular pieces of DNA—inside a cell. One plasmid carries the gene for a protein of interest and for a bioluminescent protein that acts as an energy donor. In standard BRET, the donor is Renilla luciferase, which comes from the sea pansy and gives off blue light. The second plasmid carries the gene for a protein that interacts with said protein of interest and for an energy acceptor molecule that glows when triggered by light. When a cell expresses the two proteins of interest and they interact, the donor gets close enough to excite the acceptor, and the acceptor emits light at a particular wavelength that can be detected and quantified via spectroscopy.
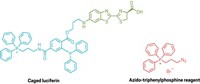
BRET gives a better picture of how proteins behave in the cell than methods that detect protein interactions in a test tube, but the signal from the standard acceptor/donor pairs can be weak, according to study author Thomas Machleidt. “You need unusually high expression levels to be able to measure a signal,” he says. To overcome this limitation, Machleidt and coworkers, in previous work, engineered a new luciferase derived from deep sea shrimp (Oplophorus) that glows more than 100 times as bright as the Renilla enzyme. The new enzyme is also half the size and therefore is less likely to interfere in protein pairings.
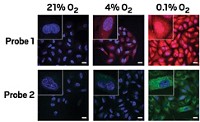
In the current study, the researchers wanted to find the new luciferase’s perfect partner, an acceptor that would give an exceptionally bright, clear signal during a protein interaction. They tested 57 acceptor fluorophores—covalently linked to a target protein via a small protein called a HaloTag, also manufactured by Promega—and found that a red-emitting fluorescent dye gave the best performance. Its signal was strong, and its emission wavelength was 175 nm away from that generated by the donor—a large gap that minimizes potential overlap with the donor signal, Machleidt says, leading to better sensitivity. These improvements, he adds, should allow researchers to monitor protein interactions in mammalian cell lines, for instance, at lower protein expression levels than have been possible with conventional BRET.
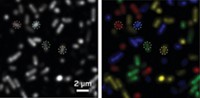
The researchers tested the new system in human embryonic kidney cells using a pair of strongly interacting, well-characterized proteins—a protein chaperone called FKBP and a signaling protein, Frb—and compared their findings with those from traditional BRET. The signal from the engineered lucerifase pair was between four- and 70-fold greater than that from conventional BRET. The increased sensitivity allowed the team to observe protein interactions in a single cancer cell using fluorescence imaging. Such an approach could allow scientists to study interactions that occur only within organelles or other subcellular compartments.
“This is a very cool technique,” says Carl H. Johnson of Vanderbilt University, one of the original developers of BRET. He particularly praised the engineering of Renilla luciferase to be both smaller and brighter. Although the Promega system may provide the highest sensitivity so far, he notes that other acceptor fluorophores should also work and be sufficiently sensitive for many applications.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter