Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Technique Uses Light To Manipulate Light-Insensitive Nanoparticles
Nanotechnology: Method can reversibly draw images in particle-laden gel
by Stu Borman
July 22, 2015
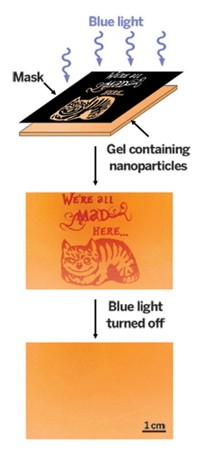
Shining blue light through a Cheshire Cat-patterned mask onto a gel containing protonated merocyanine (MCH+) and carboxylated nanoparticles, forms spiropyran (SP) and increases the acidity of the gel (mechanism below). As a result, the nanoparticles disperse (indicated by red color), creating a cat image. When the light is turned off, the particles reassemble and the image disappears in about three minutes.

Shining blue light through a Cheshire Cat-patterned mask onto a gel containing protonated merocyanine (MCH+) and carboxylated nanoparticles, forms spiropyran (SP) and increases the acidity of the gel (mechanism below). As a result, the nanoparticles disperse (indicated by red color), creating a cat image. When the light is turned off, the particles reassemble and the image disappears in about three minutes.
Manipulating nanoparticles with light isn’t unheard of. Scientists have long been illuminating and pushing around particles coated with light-activated molecules. But using light to manipulate particles without this type of responsive coating hasn’t been demonstrated previously.
Now, Rafal Klajn of Israel’s Weizmann Institute of Science and coworkers have developed an approach to reversibly draw together and disperse non-light-responsive particles in solution by shining light on them (Nat. Chem. 2015, DOI: 10.1038/nchem.2303). When placed in a gel solution, the particles can create images that disappear after light is switched off. Potential applications of the technology include nanofabrication and controlled drug delivery.
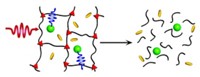
In earlier studies, researchers made nanoparticles that assemble and disperse reversibly by attaching photoresponsive ligands such as azobenzenes and spiropyrans to them. But these photoresponsive nanoparticles are often difficult to synthesize and have properties that impair particle assembly and disassembly. The new technique instead requires only that gold nanoparticles be derivatized with common carboxylic acid groups, which are inherently pH-sensitive.
Klajn and his team place these particles into a solution containing protonated merocyanine (a spiropyran derivative). The particles assemble in clusters because of hydrogen bonding between their carboxylic acid groups. Shining blue light on the solution causes protonated merocyanine to release protons and convert to spiropyran. The released ions protonate the carboxylic acid groups, blocking hydrogen bonding and causing the nanoparticles to disperse. Turning off the light causes spiropyran to convert spontaneously back to protonated merocyanine, inducing the nanoparticles to give up solitary existence and reassemble.
The researchers demonstrated the new technique by drawing images of the Cheshire Cat from “Alice’s Adventures in Wonderland.” To do so, they shined light through a lithographic mask onto a gel containing protonated merocyanine and coated nanoparticles. The images appeared when light dispersed the particles, which turn red because of plasmonic effects, and disappeared a few minutes after the light was switched off as the particles reclustered.
The study “demonstrates an efficient and convenient method for reversible photo-patterning, which is difficult to achieve and may find applications in photonic devices,” says photochemist Yi Liao of Florida Institute of Technology, in Melbourne.
Molecular materials expert Rienk Eelkema of Delft University of Technology, in the Netherlands, notes that the technique, which currently uses methanol solutions, will need to be extended to water-based solutions to be useful for many applications. In addition, Eelkema says, extending the concept to other chemical and biochemical signals besides carboxylate protonation and deprotonation could open opportunities for its use to control the movement and assembly of nanoparticles in living systems.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter