Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
ACS Meeting News: MOF Phosphor Improves On Light-Emitting Diodes
by Stephen K. Ritter
August 19, 2015
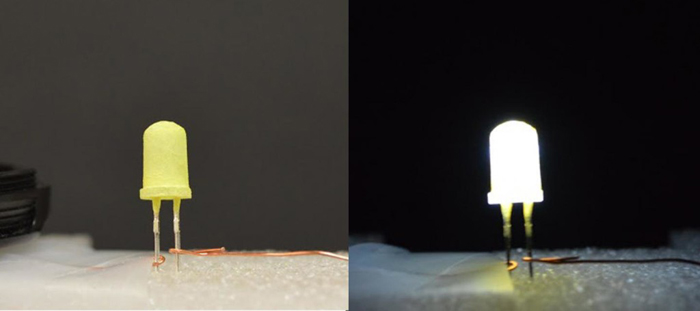
Light-emitting diodes are now state-of-the-art when it comes to lighting. Yet scientists are still designing new ways to improve on the quality of light that LEDs produce and to reduce their cost and make them environmentally friendlier. A team led by Zhichao Hu and Jing Li of Rutgers University has hit on all those goals in its design of a new white LED.
“LEDs are everywhere, making their way into our homes, offices, and schools to replace fluorescent and incandescent lights,” Hu said. “But it’s not easy to make white LEDs. We make what’s called a phosphor-converted LED by coating an LED of one color with a phosphor material of a different color.”
Hu reported the work Wednesday during a symposium organized by the Division of Inorganic Chemistry at the American Chemical Society national meeting in Boston.
The researchers started by developing a family of tetraphenylethylene-based chromophores that luminesce at different wavelengths depending on placing different functional groups on the phenyl rings, Hu explained. They then linked chromophores together with zinc atoms to form a three-dimensional metal-organic framework. Immobilizing the chromophores helps tune and stabilize their color emission.
The Rutgers researchers can make any color LED they want by coating different colored LEDs with the different phosphors. The phosphor is excited by light produced by the underlying LED, and the mixture of the emissions from the LED and phosphor produce the desired color of light. To make a white LED, they coated a blue LED with a yellow phosphor named H4tcbpe. The new material has a performance level approaching that of the best commercially available phosphors, Hu said. In addition, it doesn’t require an increasingly expensive rare-earth metal typically used in LED phosphors and avoids toxic cadmium selenide used in quantum dot-based LEDs. The Rutgers researchers have patented the technology and are testing the materials with commercial LED manufacturers.
Current inorganic luminescent materials can be very efficient and stable, but their luminescence properties often have limited tunability, commented Oleg Shchekin, senior director of device architecture at Philips Lumileds, a leading LED producer. The freedom to tune emission spectra, as the Rutgers team is doing, is therefore important for maximizing the efficiency of LEDs, Shchekin says. “This work is showing the feasibility of hybrid materials, where the inorganic component is leveraged for efficiency while the emission from the organic component is tuned over a wide range.”
Li’s group at Rutgers has been a leader in developing framework-based luminescent materials,” added Ram Seshadri, codirector of the Materials Research Laboratory at the University of California, Santa Barbara. “A particularly exciting aspect of these newly developed materials lies in the ease with which they can be painted on flexible substrates, suggesting unusual lighting sources and geometries are possible.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter