Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Determining The Estrogenic Activity Of BPA Replacements
Toxicology: Computer model identifies 11 bisphenol A substitutes predicted to have similar ability to bind to estrogen receptors
by Puneet Kollipara
September 16, 2015

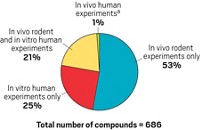
Long used in polycarbonate plastics, epoxy resin food-can liners, and thermal receipt paper, bisphenol A (BPA) has become a poster child for endocrine disruptors—compounds that mimic or interfere with the body’s hormones, such as estrogens and androgens. Many manufacturers have replaced BPA with alternatives in recent years, but dozens of BPA substitutes lack data on their potential estrogenic activity. Now, a new computer model provides a way to determine whether a BPA substitute shows high potential to bind the estrogen receptor and requires further testing for its endocrine disrupting effects (Chem. Res. Toxicol. 2015, DOI: 10.1021/acs.chemrestox.5b00243).
Huixiao Hong of the Food & Drug Administration’s National Center for Toxicological Research and his colleagues developed a model that assesses how well a compound orients itself with the estrogen receptor, and then estimates binding affinity by calculating how energetically favorable the binding is. The researchers trained the model by running it on 18 BPA substitutes that already have estrogenic activity data and two reference compounds: BPA itself and 17β-estradiol, a key female sex hormone.
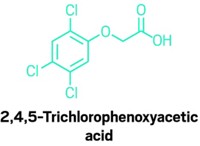
When the researchers ran the model on 27 BPA substitutes that lacked experimental data on estrogenic activity, the model identified 11 that met the criteria for binding to the receptor. Many compounds were predicted as better potential binders than BPA, including tetrabromobisphenol A, used in plastics and as a flame retardant, and bis-(3-allyl-4-hydroxyphenyl)sulfone, used in thermal receipt paper.
Binding the receptor alone isn’t sufficient to make the compound an endocrine disruptor, the researchers note. But they add that the findings do suggest the need for additional studies of these compounds’ safety.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter