Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Copolymer Helps Make Flexible Li-Ion Battery Electrodes
Electrochemistry: Conductive block copolymer improves vanadium pentoxide cathodes’ mechanical and electrochemical properties
by Mitch Jacoby
October 2, 2015

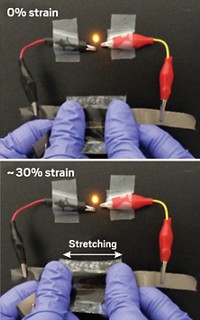
Flexible, stretchable electronics—a concept that until recently seemed radical—is starting to turn objects such as clothing into powerful gadgets. These devices, which could monitor and relay information about a wearer’s health, for example, could become more widely available if there were thin, flexible batteries to power them. But most batteries are bulky and rigid and their components are often brittle.
Researchers have taken a step toward overcoming that obstacle by hybridizing a candidate for lithium-ion battery electrodes, V2O5, with a conductive polymer. Not only does that process convert the electrode material from a paperlike form that easily cracks to one that’s flexible and robust, it also improves its electrochemical properties (Sci. Rep. 2015, DOI: 10.1038/srep14166).
V2O5 has caught the interest of battery researchers because the inexpensive oxide has the potential for a high charge capacity due to its ability to store and release a lot of lithium. Also it is easily formed as a paperlike material, which is key for making electrodes. But the material conducts ions and electrons sluggishly and it’s prone to swelling and cracking as it charges and discharges.
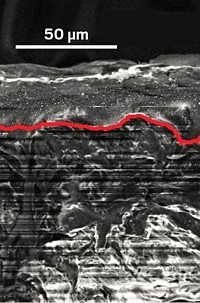
To address those shortcomings, a research team led by Texas A&M University chemical engineers Hyosung An, Jared Mike, and Jodie L. Lutkenhaus used a simple water-based process to blend V2O5 with poly(3-hexylthiophene)-block-poly(ethyleneoxide), P3HT-b-PEO, a block copolymer composed of electron- and ion-conducting units. The researchers developed the blending process mindful of the tradeoffs in making hybrid electrode materials. Such hybrids sometimes can improve mechanical properties of the materials at the expense of electrochemical ones. In addition, adding polymer reduces the concentration of V2O5 and hence reduces the Li-ion storage capacity.
So the team sought to keep the polymer concentration low. They found that adding just 5 wt % polymer led to a hybrid that’s three times as flexible and twice as tough as pure V2O5 Hybridizing also roughly doubled the lithium-ion diffusion, eliminated cracking during battery charging and discharging, and slowed the battery’s loss of capacity over time. The team attributes the enhancements to the hybrid material’s unique self-assembled structure, which consists of interlocking V2O5 layers held together by micellar aggregates of P3HT-b-PEO.
“The electrode fabrication described here is novel,” says University of California, Berkeley, chemical engineer Nitash P. Balsara, a specialist in block copolymer battery applications. Balsara adds that he is impressed with the way the team integrated this polymeric electrode with conventional battery components. “In doing so, Lutkenhaus and her collaborators have assembled a very interesting electrochemical energy storage device.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter