Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Tiny Boxes Hold Catalytic Surprise
Materials: Cages made from nanoparticles could challenge dominance of ELISA enzyme
by Neil Savage
October 30, 2015

Researchers have been exploring ways to build tiny containers out of nanoparticles; such superstructures can help deliver drugs to specific tissues, hold molecules that act as biological sensors or catalyze reactions, and make it easier for microscopes or MRI machines to image tiny targets. Now, one group has come up with an easy way to build nanoparticle cages, and to their surprise, the boxes are much more efficient catalysts than an enzyme commonly used in ELISA, the popular immunoassay for detecting many types of biomolecules (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b09337).
Weihong Tan, a chemist at the University of Florida and Hunan University, in China, and his group were working to create large-surface-area nanostructures that could hold and deliver high doses of drugs. Existing processes for making such superstructures are time consuming and complex, and the researchers were hoping their approach would be simpler.
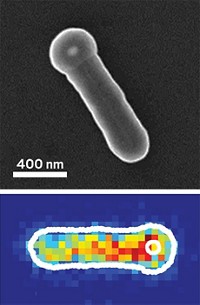
The team started by making copper hydroxide nanoparticles, which they dispersed in water with polyvinylpyrrolidone. Next, they added a copper-ammonia complex to the solution, triggering a series of reactions that led to migration of the copper hydroxide into one-dimensional “nanoribbons” that formed evenly spaced bars surrounding the nanoparticles. Eventually the 200- to 250-nm particles completely disappeared, leaving behind a self-assembled three-dimensional cage of the same size with a hollow center. “The formation is very regular, and it gives you a uniform structure,” Tan says. He does not yet have an explanation for the mechanism leading to that structure.

The group tested the material’s catalytic activity and found that it had high activity for the colorimetric reaction typically catalyzed by the horseradish peroxidase enzyme. This reaction is central to many biological assays, especially the widely used ELISA, or enzyme-linked immunosorbent assay, used to detect the presence of antigens in samples. In the presence of the group’s cages, a solution containing the reagents tetramethylbenzidine and hydrogen peroxide changed from clear to deep blue within five minutes at room temperature. The same reaction with horseradish peroxidase took longer, even though the concentration of the enzyme was 2,000 times that of the copper hydroxide cages. Tan says he needs to do more work to figure out how the system works. “I don’t really know why we have all this catalytic activity,” he says.
Jianfang Wang, a professor of physics who studies nanoparticles at the Chinese University of Hong Kong, is impressed with the work. “I am very surprised by the fact that such complex nanoarchitectures can be synthesized by such a simple wet-chemistry method in aqueous solutions,” he says. “This is probably the most complex structure that I have ever seen among those made by wet-chemistry methods.”
He would like to know more about the mechanism that causes the cages to form, as well what causes the high catalytic activity. He’s also interested in learning how selective the material is. “Selectivity is probably more important than activity for enzymes,” Wang says.
Tan says his next step is to see whether he can make a complete assay that might be able to replace ELISA.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter