Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
How A Switchable Polymer Could Combat Antibiotic Resistance
Pacifichem News: Material kills bacteria only when necessary, thanks to reversible supramolecular chemistry
by Matt Davenport
December 16, 2015

As infectious bacteria continue to evolve defenses against conventional antibiotics, many people are focused on the need for more potent drugs.
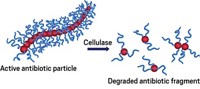
But Shu Wang of the Chinese Academy of Sciences presented a different route for fighting antibiotic resistance on Tuesday during the 7th International Chemical Congress of Pacific Basin Societies, or Pacifichem. Wang and his team have developed polymers with antimicrobial activity that can essentially be turned on and off (Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201504566).
The polymers are actively antimicrobial only when they need to be so they don’t continually pressure bacteria to evolve resistance, Wang says.
Although making switchable antibiotics isn’t a new strategy, Wang and his team believe they have a fresh, promising approach. Previously, researchers have investigated compounds that can be switched on or off using light. Wang’s antibiotics use reversible supramolecular chemistry that also works in the dark, he tells C&EN.
These biocides are cationic polymers derived from poly(phenylene vinylene). The polymers have linear backbones with cationic ammonium arms that control the material’s antibiotic properties. The positively charged arms help kill bacteria by penetrating into cell membranes. This behavior is governed primarily by electrostatics, although hydrophobic interactions may also contribute, Wang says.
He adds that the polymers are designed to attack bacterial cells and not mammalian cells, including healthy human cells.
To switch off the antimicrobial activity, Wang’s team exposes the polymers to ring-shaped cucurbit[7]uril molecules, or CB[7]. These compounds cuff the ammonium arms, hindering the polymer’s ability to latch on to bacteria.
To restore the polymer’s antimicrobial activity, the team adds the small molecule amantadine to the bacterial system, usually a culture of E. coli. Amantadine bonds with CB[7], removing the cuffs from the polymers, Wang explains.
This is an interesting approach, says Ben L. Feringa of the University of Groningen, who was not involved in this study but pioneered earlier examples of switchable antibiotics. When it comes to combatting antibiotic resistance, “innovative approaches should be applauded,” Feringa adds. Yet he says he has a hard time imagining how this multiple-component process would be administered to patients in the clinic.
Wang is optimistic, however, and he points out that the polymers could also be used to deactivate antimicrobials before they are released into the environment, for instance, in wastewater from agriculture. “Antibiotic resistance is a very big problem for society,” he says. “We think this could be a fast way to reduce that trouble.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter