Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Light sparks conversion of dinitrogen to ammonia
Hybrid nanotech-biological technique could lead to an environmentally friendly route to nitrogen fixation
by Stu Borman
April 22, 2016
| A version of this story appeared in
Volume 94, Issue 17

In an effort to improve on the way both nature and industry run chemical reactions, researchers have developed a technique that uses light to spur a bacterial enzyme to convert dinitrogen (N2) into ammonia (NH3). The method might one day lead to a more environmentally friendly industrial process for making NH3.
Currently, the iron-catalyzed Haber-Bosch process, used to make NH3 from N2, is one of the world’s largest industrial processes in terms of tonnage produced. Ammonia is a major ingredient of fertilizer, and in the past century, the Haber-Bosch process has boosted crop production so much that the world population would be unsustainable without it. Ammonia is also a potential energy source for NH3-based fuel cells that power motor vehicles.
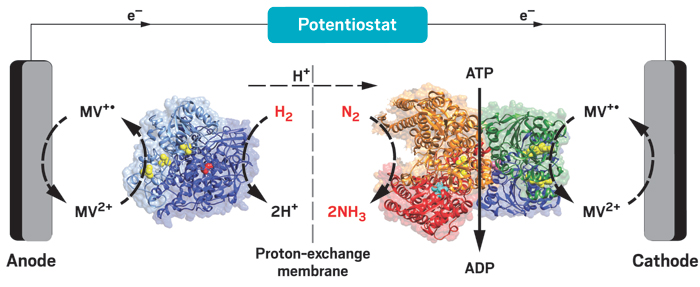
But the Haber-Bosch process requires high temperatures and pressures, is powered by fossil fuels, and emits large amounts of the greenhouse gas carbon dioxide. Nitrogen-fixing bacteria carry out the same process at room temperature with nitrogenase enzymes, but the eight-step biological process is slow and requires large amounts of the cellular fuel adenosine triphosphate (ATP).
Researchers have been trying for years to harness the best of both worlds by developing ways to combine the high efficiency of the Haber-Bosch process with the mild conditions, low greenhouse gas emissions, and reduced fossil-fuel dependence of biological nitrogen fixation. Now, Paul W. King of the National Renewable Energy Laboratory and coworkers have come up with a potentially fruitful approach (Science 2016, DOI: 10.1126/science.aaf2091).
They tested various redox-active nanocrystals before coming up with a cadmium sulfide (CdS) nanorod that efficiently transfers electrons to one of the nitrogenase complex’s two proteins—molybdenum-iron (MoFe) protein, which uses electrons to power N2-to-NH3 conversion. The nanorod replaces nitrogenase’s other protein, Fe protein, by substituting light-driven energy for Fe protein’s ATP-driven energy generation process. The hybrid technique could use solar power to reduce N2 to NH3 and would produce no CO2. It works 63% as fast as native nitrogenase right now. But it might never reach the speed of the Haber-Bosch process because even native nitrogenases are slow in comparison.
Even so, biohybrid catalysis could be an effective route “to solar-driven reduction of small molecules such as N2 and CO2, reactions that are traditionally difficult to activate,” says nanocrystal catalysis specialist Peidong Yang of the University of California, Berkeley. “It takes advantage of the best of two worlds: the great light-harvesting capability of synthetic semiconductor nanostructures and the catalytic power of biology.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter