Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
The first phosphaphosphenium cation isolated
Chemists prepare HP2+, the elusive phosphorus analog of the diazonium cation
by Stephen K. Ritter
April 25, 2016
| A version of this story appeared in
Volume 94, Issue 17
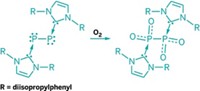
Researchers who spend their time seeking out strange new molecules made from main-group elements have enjoyed great success in recent years thanks to the discovery of N-heterocyclic carbenes (NHCs). These Lewis bases with a sturdy lone pair of electrons can bind and stabilize metals and molecule fragments in ways not possible before. In the latest example, Yuzhong Wang, Gregory H. Robinson, and coworkers of the University of Georgia have used the NHC-stabilization strategy to prepare the parent phosphaphosphenium cation, HP2+, for the first time. The complexity of the cation’s name hints that chemists have had a complex time isolating the deceptively simple species, previously only detecting it in gas-phase experiments. To make HP2+, Robinson and coworkers used pyridine hydrochloride (HCl•NC5H5) to protonate NHC-stabilized diphosphorus, P2, another compound they previously tamed. Unlike its nitrogen analog, N2, diphosphorus is unstable. The Georgia team further obtained the X-ray crystal structure of NHC-stabilized HP2+ and conducted spectroscopic and computational bonding analyses (Chem. Commun. 2016, DOI: 10.1039/c6cc0175b). The findings could lead to better understanding of how to use diphosphorus in organic synthesis and materials science, for example, similar to how aryl diazonium cations RN2+ are used in palladium-catalyzed cross-coupling reactions.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter