Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Iron-sulfur gel provides possible green route to ammonia
Chalcogel catalyst reduces dinitrogen in water under ambient temperature and pressure
by Stu Borman
May 12, 2016
| A version of this story appeared in
Volume 94, Issue 20
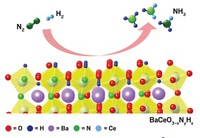
![Reaction scheme shows how a newly developed chalcogel, a network of Fe4S4 clusters linked with [Sn2S6]4- anions, uses visible light energy to convert N2 to NH3. Reaction scheme shows how a newly developed chalcogel, a network of Fe4S4 clusters linked with [Sn2S6]4- anions, uses visible light energy to convert N2 to NH3.](https://s7d1.scene7.com/is/image/CENODS/09420-notw6-boxstruct-700?$responsive$&wid=300&qlt=90,0&resMode=sharp2)
To produce the large quantities of fertilizer needed to sustain global food production, industry relies on the Haber-Bosch process to split dinitrogen from the air to synthesize ammonia. But to generate the high temperatures and pressures needed for the reaction, ammonia plants consume fossil fuels and, as a result, contribute significantly to greenhouse gas emissions.
Researchers want to develop a green alternative to the Haber-Bosch process. In a new effort, Mercouri G. Kanatzidis and George C. Schatz of Northwestern University and coworkers used iron-sulfur (Fe4S4) clusters, found naturally in the nitrogenase enzymes bacteria use to split dinitrogen, to make a light-driven catalyst that converts N2 to NH3 in water at ambient temperature and pressure (Proc. Natl. Acad. Sci. USA 2016, DOI: 10.1073/pnas.1605512113).
After synthesizing the Fe4S4 clusters, the researchers link them with [Sn2S6]4– anions and form the complex into a chalcogel, a foamy gel containing the chalcogen element sulfur. The black material absorbs visible light and uses that energy to break the N≡N triple bond, one of the strongest in chemistry. The nitrogen atoms then combine with water-derived hydrogen to make NH3.
Other researchers have developed transition metal-N2 complexes that convert N2 to NH3, but those systems typically require organic solvents, low temperatures, and/or high pressures. Some previously developed light-based systems also can reduce N2, but the chalcogel is 10 times as efficient
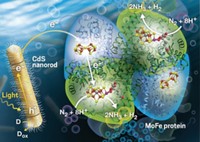
Hybrid synthetic-biological approaches, such as a nanorod-nitrogenase system (Science 2016, DOI: 10.1126/science.aaf2091, C&EN, April 25, page 9), are much more efficient than the chalcogel system. But the hybrid approach has limitations: Obtaining nitrogenase from organisms is difficult, and the enzymes are not stable for more than a few hours after isolation, Kanatzidis says.
The chalcogel is orders of magnitude less efficient than the Haber-Bosch process. But unlike the Haber-Bosch process or metal-N2 complexes, the chalcogel catalyst can fix N2 under quite mild conditions using light as the only driving force, comments photocatalysis specialist Lizhi Zhang of Central China Normal University. Understanding of the chalcogel mechanism remains fragmentary at this point, he says, so further study is still needed to find ways to optimize the catalyst.
Catalysis expert Patrick Holland of Yale University says it isn’t fair to compare the efficiency of the chalcogel to the Haber-Bosch method because the industrial process has been optimized for more than a century. “The chalcogel already makes reasonable amounts of ammonia, so it’s a valid first step toward using light to drive N2 reduction,” he says. “The catalyst is very modifiable, so it could in principle be improved a lot in the long run.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter