Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
For radical enzyme catalysis, an organometallic intermediate is pinpointed
An iron-sulfur cluster may bind 5′-deoxyadenosyl in enzymes that use S-adenosylmethionine
by Jyllian Kemsley
May 16, 2016
| A version of this story appeared in
Volume 94, Issue 20
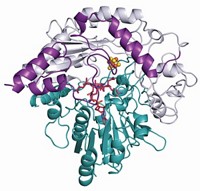
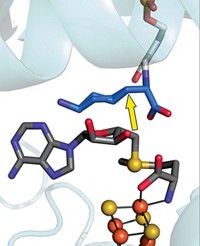
Synthesizing heme and some vitamin and antibiotic compounds requires enzymes that mediate radical chemistry. But enzymes must carefully control radical compounds, lest they escape and wreak havoc within a cell. A study suggests a new role for an iron-sulfur cluster in one superfamily of 100,000 enzymes that use S-adenosylmethionine (SAM) to catalyze radical reactions. Researchers knew from earlier studies that the cluster cleaves SAM to produce 5′-deoxyadenosyl radical (5′-dAdo•). Now, they have discovered that the cluster also binds 5′-dAdo through an Fe–C bond (Science 2016, DOI: 10.1126/science.aaf5327). A team led by Joan B. Broderick of Montana State University and Brian M. Hoffman of Northwestern University trapped a reaction intermediate of pyruvate formate-lyase activating enzyme, which uses SAM to generate a glycyl radical on the target lyase. They used electron nuclear double resonance spectroscopy to study the intermediate and found that it is an organometallic species, with a covalent link between the 5′-C of 5′-dAdo and an iron atom of the cluster. Although it is unclear whether other radical SAM enzymes follow the same mechanism, this scheme echoes that of enzymes that use vitamin B-12 (adenosylcobalamin) to conduct radical chemistry.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter