Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chiral tertiary alcohols forged with copper
Catalytic asymmetric addition of olefin-derived nucleophiles to ketones creates valuable motif
by Bethany Halford
June 13, 2016
| A version of this story appeared in
Volume 94, Issue 24
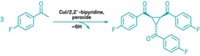
To make a tertiary alcohol from a ketone, chemists will often use an organometallic reagent to muscle its way onto the ketone’s carbonyl carbon. But there are limitations to which substrates you can use in this reaction, because the organometallics will react with substituents other than the target carbonyl. Also, to do the reaction asymmetrically, which is desirable to make drug candidates, for example, chemists often have to add stoichiometric amounts of chiral additives. Seeking a method that overcomes these burdens, a team led by MIT’s Stephen L. Buchwald and the University of Pittsburgh’s Peng Liu has developed an asymmetric tertiary alcohol synthesis that uses catalytic amounts of copper hydride and a chiral phospholane ligand to couple a polyunsaturated hydrocarbon, such as an ene-yne, and a ketone (Science 2016, DOI: 10.1126/science.aaf7720). The reaction (one example shown) tolerates a range of coupling partners and proceeds in high yield and with high enantioselectivity. In many instances, the chemists built complex molecules with two adjacent chiral centers. With further ligand optimization, the reaction will become “a powerful tool for the asymmetric addition of olefin-derived nucleophiles to carbonyls that will be of broad synthetic utility,” the researchers believe.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter