Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Catalyst combo could oxidize biomass alcohols
Electrochemical alcohol oxidation is more efficient with two catalysts than one
by Stu Borman
July 4, 2016
| A version of this story appeared in
Volume 94, Issue 27
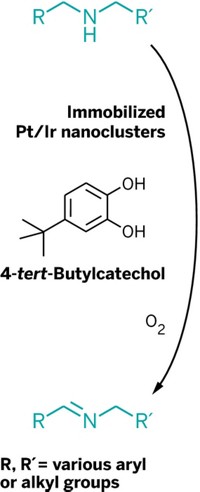
A pair of catalysts can oxidize alcohols electrochemically in a relatively efficient and speedy manner. Such a catalyst system could find use in fuel cells powered by biomass. Organic alcohols are abundant in biomass derived from trees and other plants, and fuel cells could generate electricity by oxidizing such alcohols electrochemically. TEMPO (2,2,6,6-tetramethyl-1-piperidine N-oxyl) is an effective catalyst for such oxidations, but it requires running fuel cells at high electrode potentials, which is not energy efficient. Shannon S. Stahl and Artavazd Badalyan at the University of Wisconsin, Madison, have now developed a dual electrocatalyst system that overcomes this problem (Nature 2016, DOI: 10.1038/nature18008). They show that (2,2′-bipyridine)Cu(II) and TEMPO work cooperatively as catalytic redox partners in a two-electron oxidation of alcohols. The two-catalyst oxidations run at a more-efficient electrode potential—a reduction of a half-volt—and are nearly fivefold faster, compared with TEMPO-only reactions. Electrocatalysis expert Shelley D. Minteer of the University of Utah says that the next steps toward incorporating the cocatalysts in a fuel cell are demonstrating their long-term stability and that they can be immobilized on surfaces.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter