Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Presenting a ferrocene Ferris wheel
Fused ferrocene units form redox-active nanorings that offer possibilities for host-guest, electronic, and magnetic applications
by Stephen K. Ritter
July 4, 2016
| A version of this story appeared in
Volume 94, Issue 27
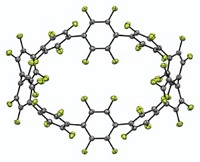
By fusing ferrocene molecules together, a research team has prepared new iron-based macrocycles that resemble a Ferris wheel. Ferrocene linear chains and macrocycles have been made before, but they contained spacer groups separating the metallocene units, which don’t allow much interaction between the iron atoms. Michael S. Inkpen, Nicholas J. Long, and Tim Albrecht of Imperial College London and their colleagues have now made examples in which five to nine ferrocene units are fused by direct cyclopentadienyl C–C linkages. These redox-active ferrous/ferric nanorings have substantial charge delocalization and are more stable than previous ferrocene-based macromolecules, with the six-membered version representing an organometallic analog of benzene (Nat. Chem. 2016, DOI: 10.1038/nchem.2553). The researchers made the ferrocene rings via copper-mediated Ullmann coupling reactions of dilute solutions of iodinated ferrocene or linear ferrocene oligomers. The internal cavity of the molecules provides opportunities for host-guest chemistry, and the charge delocalization could lead to electronic and magnetic applications, the researchers say. The team is now investigating more efficient synthetic routes to the macrocycles and methods for derivatizing the rings and potentially linking them together to form large-scale arrays.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter