Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Helical polyiodide chains resolve starch test mystery
Researchers find that infinite iodide strands provide structure and electronic balance for starch as well as polycyclic organic conductors
by Stephen K. Ritter
July 11, 2016
| A version of this story appeared in
Volume 94, Issue 28
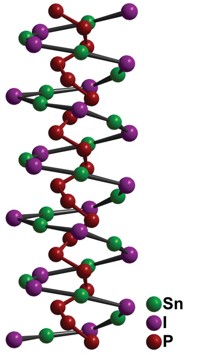
In the course of developing new semiconducting materials, a research team including Ram Seshadri and Fred Wudl of the University of California, Santa Barbara, has solved a historical puzzle regarding the structure of the starch-iodine complex (Angew. Chem. Int. Ed. 2016, DOI: 10.1002/anie.201601585). Iodine dissolved in potassium iodide solution has long been used as a test for the presence of starch. The helical iodide chain that forms wraps around starch molecules (amylose) to create a complex that produces a deep purple color. Although scientists have speculated that polymeric iodide chains are involved, the exact structure has remained elusive. Chemists have reported other organic polyiodide complexes, but when characterized they have been found to have chain lengths shorter than 10 iodide units and contain molecular iodine (I2) within the chain. The perylene-iodine complex, for example, made up of stacks of the planar polycyclic aromatic hydrocarbon perylene interspersed with iodide strands, was one of the first known organic conducting materials. But like the starch-iodine complex, its structure has never been fully resolved either. Seshadri, Wudl, and coworkers prepared a similar conductive material made from an iodine solution with pyrroloperylene and were able to get a crystal structure. The team concludes that stacks of pyrroloperylene molecules are interspersed with helical polyiodide strands of infinite length with no neutral I2 units. The researchers have shown that the starch-iodine complex also contains these extended polyiodide chains.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter