Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Novel strategy finds long-sought inhibitor for cancer-related enzyme
After two decades of effort, researchers identify clinically viable agent that blocks SHP2’s cancer-promoting activity
by Stu Borman
July 7, 2016
| A version of this story appeared in
Volume 94, Issue 28

A novel strategy has broken a two-decade impasse in efforts to find clinically developable drug inhibitors of SHP2, a phosphatase enzyme that plays an important role in many cancers.
Activation of SHP2 by proteins overexpressed in cancer, such as endothelial growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2), promotes tumor growth. But SHP2 helps stimulate cancer growth only when it adopts an open conformation, in which its three domains fan out and its active site becomes exposed.
In 20-odd years of efforts to inhibit SHP2, researchers have tried to target the active site in the open conformation. The problem is that the negatively charged compounds they’ve used to inhibit SHP2’s polar active site do not make good drugs because they are not able to cross cell membranes easily or enter the bloodstream effectively when administered orally. So those SHP2 inhibitors haven’t worked well therapeutically, and none have entered clinical trials.
A drug discovery group led by William R. Sellers, Travis Stams, and Pascal D. Fortin and a medicinal chemistry team led by Matthew J. LaMarche at Novartis Institutes for BioMedical Research have now sidestepped that problem (Nature 2016, DOI: 10.1038/nature18621 and J. Med. Chem. 2016, DOI: 10.1021/acs.jmedchem.6b00680). Instead of inhibiting the active site in SHP2’s open conformation, the researchers keep SHP2 completely closed. When SHP2 is closed, its active site is inaccessible and incapable of helping promote cancer growth.
The Novartis team screened a compound library to find an agent that prevented SHP2 from opening. They initially found hits with only weak affinity and then used structure-based drug design to optimize candidates for affinity, cell permeability, and properties that enable oral administration.
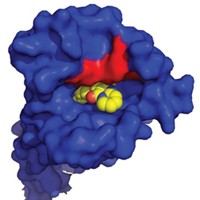
The result was a compound called SHP099, which strongly binds SHP2. It can be administered orally and kills cancer cells in mice with implanted tumors. In a crystallography study, the researchers discovered that it occupies a tunnel-like binding site in SHP2’s closed conformation. In that strategic location, it is able to glue together all three of the enzyme’s domains and prevent them from separating.
“Nobody has seen this tunnel before,” says SHP2 specialist Walter Birchmeier of the Max Delbrück Center for Molecular Medicine. Blocking SHP2 this way is thus “a revolutionary new concept,” he says.
On the down side, Birchmeier also speculates that SHP099 could cause significant side effects by binding tunnels or ion channels in other key proteins that control heart and brain functions. But the Novartis team found that the compound was specific for SHP2 over other phosphatases—including SHP1, the most similar one—and had negligible activity in preclinical tests against possible off-target proteins, including several ion channels and G-protein coupled receptors. “From our perspective, SHP099 is looking quite promising and selective,” Stams says. The group has filed for patents and is optimizing SHP099 for testing in patients.
It’s not clear yet if the new inhibitor strategy will work well in people, comments pharmaceutical and medicinal chemist Jörg Rademann of the Free University of Berlin. But, he adds, SHP099’s high cell permeability could be a major advantage, and previous findings suggest that SHP2 inhibition could actually prevent cancer in addition to treating existing cases.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter