Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
New tetradentate ligand exposes metal
Pocket structure and electronic properties could lead to new chemistry
by Elizabeth K. Wilson
July 18, 2016
| A version of this story appeared in
Volume 94, Issue 29
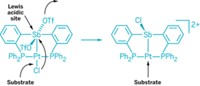
Chemists have synthesized the first tetradentate ligand containing three phosphaalkenes, which, in complexes with rhodium and iridium, forms binding pockets that could lead to new chemistry at the metal site (Organometallics 2016, DOI: 10.1021/acs.organomet.6b00250). A team led by chemistry professor Matthew F. Cain of the University of Hawaii, Manoa, synthesized the tris(phosphaalkene)phosphine ligand in a single step from an ortho-substituted triphenylphosphine scaffold. Each phosphaalkene group contains a bulky substituent, 2,4,6-tri-tert-butylbenzene. When complexed with Rh or Ir, the ligand closes around the metal to form a cup-shaped structure. The combination of a pocket that harbors an exposed metal site and low-lying π* orbitals creates an unusual steric and electronic environment, which could allow chemistry with typically unreactive species, the authors say.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter