Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
There’s plenty of nitrogen to go around in cyclopentazole anion
Chemists make and purify an elusive all-nitrogen aromatic ring
by Bethany Halford
July 18, 2016
| A version of this story appeared in
Volume 94, Issue 29
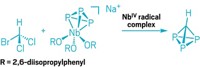
Nitrogen, unlike its next-door neighbor carbon, is not known for its propensity to form long chains or multiatom rings. But when strung together in these ways, polynitrogen compounds have applications as high-energy materials because they can break apart with explosive results. One such polynitrogen compound—the cyclopentazole anion—has long been sought because it is thought this molecule might be somewhat stable, thanks to its aromaticity. Previous studies identified the all-nitrogen five-membered ring in the gas phase, but no one has produced and directly detected the anion in solution—until now. Chemists at Hebrew University of Jerusalem, led by Yehuda Haas, report they were able to prepare the cyclopentazole ion by reducing phenylpentazole with sodium (shown) using tetrahydrofuran as a solvent. The researchers propose this produces the phenylpentazole radical anion, which then decomposes into the phenyl radical and cyclopentazole anion (Angew. Chem. Int. Ed. 2016, DOI: 10.1002/anie.201605400). The cyclopentazole anion may be used in subsequent transformations, Haas and coworkers say. It is indefinitely stable at temperatures below –40 °C and even sticks around for a few minutes at room temperature.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter