Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Mixed-metal oxide shows promise as zinc-ion battery cathode
Zn-V2O5 material intercalates zinc ions in rechargeable aqueous grid-storage battery
by Mitch Jacoby
September 5, 2016
| A version of this story appeared in
Volume 94, Issue 35
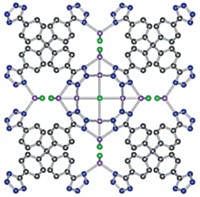
The projected low cost and inherent safety of rechargeable aqueous zinc-ion batteries make energy storage devices based on the technology seem like a real winner. Large stationary versions of such batteries could be particularly useful for grid storage applications involving intermittent power sources such as wind turbines and solar panels. But the batteries haven’t gotten off the ground yet, mainly because of unsatisfactory cathodes, which cause the devices to act sluggishly and die quickly. University of Waterloo researchers led by Linda F. Nazar have now come up with a layered vanadium oxide featuring interlayer zinc ions and water molecules that seems to bypass those problems (Nat. Energy 2016, DOI: 10.1038/nenergy.2016.119). The team used a microwave-driven hydrothermal method to synthesize ribbonlike single crystals of Zn0.25V2O5•nH2O (n = 0.85–1.0) and fashioned cathodes from thin films of the material. They paired the cathodes with metallic zinc anodes to make test cells. Analyses based on X-ray diffraction and other methods show that on discharge Zn2+ ions from the anode intercalate reversibly into the cathode, reducing the oxide and displacing water molecules. The team reports that test cells exhibit kinetic properties comparable with commercial lithium-ion battery cathodes and retain 80% of their charge capacity during 1,000 charging cycles.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter