Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
DuPont, ADM Unveil Route To Biobased Polyester
Renewable Chemistry: Low-cost method for making key monomer enables a sugar-derived plastic for bottles
by Alexander H. Tullo
January 25, 2016
| A version of this story appeared in
Volume 94, Issue 4

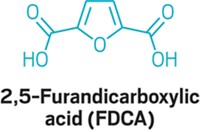
DuPont and agriculture giant Archer Daniels Midland have unveiled a process to make furan dicarboxylic methyl ester (FDME) from fructose. The companies plan on reacting the chemical with 1,3-propanediol to make a new biobased packaging polymer.
FDME is the methyl ester of furandicarboxylic acid (FDCA), which the Department of Energy earlier identified as an important potential biobased chemical building block.
The Dutch firm Avantium has been promoting FDCA as a raw material to make polyethylene furanoate (PEF), which it bills as a biobased alternative to the conventional packaging polyester polyethylene terephthalate (PET).
DuPont is developing a different polymer, polytrimethylene furandicarboxylate (PTF), which is synthesized by reacting FDME with 1,3-propanediol made at its Tennessee joint venture with Tate & Lyle. Like PEF, PTF boasts better gas barrier properties than PET.
In addition to packaging applications such as beverage bottles, ADM and DuPont say, they see potential for their new polymer in fiber and engineering plastic applications.
FDME can furthermore form PEF when reacted with ethylene glycol.
DuPont and ADM say they were working independently on a route to FDME and combined their efforts about three years ago. The process they developed uses chemical catalysis to convert fructose and methanol into FDME. DuPont says the setup is lower-cost than existing processes to make FDCA.
The two companies plan to build a 60-metric-ton-per-year demonstration facility at ADM’s complex in Decatur, Ill. Avantium, meanwhile, has been working with Coca-Cola and Danone to develop PEF. The company intends to detail its plans for its first commercial-scale FDCA plant later this quarter.
William Tittle, a principal at the consulting firm Nexant, says PEF and PTF are superior polymers to PET because of their strong barrier properties. However, a new polymer could end up contaminating the healthy recycling system for PET bottles. “The bugaboo is, what do you do about recycling?” he says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter