Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biochemistry
Artificial metalloenzyme is most efficient ever
Designer enzyme is a fast stereoselective carbene-insertion catalyst
by Stu Borman
October 7, 2016
| A version of this story appeared in
Volume 94, Issue 40

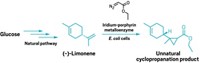
A new artificial metalloenzyme has broken an efficiency record: It’s 1,000 times as efficient as previous artificial metalloenzymes in carrying out non-biological reactions. It’s also the first that’s about as efficient as some natural biological enzymes, even though it catalyzes stereoselective organic reactions that natural enzymes can’t handle. The synthetic enzyme could lead to artificial catalysts efficient enough to accelerate stereoselective organic reactions on an industrial scale.
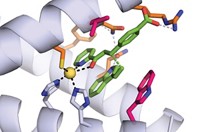
Natural enzymes have highly evolved active sites that catalyze reactions very quickly and highly selectively. Starting with a natural enzyme, John Hartwig and coworkers at the University of California, Berkeley, used active-site metal replacement and an iterative modification and selection process called directed molecular evolution to develop the new version (Science 2016, DOI: 10.1126/science.aah4427).
They created it using a strategy they reported just a few months ago (Nature 2016, DOI: 10.1038/nature17968 and C&EN, June 20, page 6). In that study, they introduced the idea of changing the active-site metal of a metalloenzyme like myoglobin and then subjecting the modified protein to directed evolution.
This time, instead of using myoglobin, the team started with a thermophilic cytochrome P450 called CYP119. Hartwig and coworkers noted that CYP119’s binding site, which is larger and more hydrophobic than myoglobin’s, accommodates organic molecules, whereas native myoglobin binds dioxygen. And CYP119 has relatively high thermal stability, so they predicted that modified versions would be stable enough to catalyze reactions quickly and repeatedly.
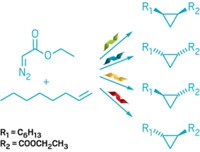
The researchers therefore replaced CYP119’s active-site iron with iridium and used rounds of directed evolution to create revised structures. The best performing metalloenzyme that emerged from the modification process is called Ir(Me)-PIX CYP119-Max. It catalyzes carbene insertion into activated C–H bonds (adjacent to electron-donating oxygen atoms), unactivated C–H bonds, and sterically hindered C–H bonds. It works at 2,550 reaction cycles per hour, comparable to the efficiency of natural enzymes. It is stereoselective, generating enantiomeric excesses up to 98%. It has good longevity of action, catalyzing 35,000 reaction cycles before running out of steam. And it works well when immobilized on a solid support, which could be useful industrially.
Its favorable properties mean small amounts of the catalyst might be practical for industrial reactions, comments artificial metalloenzyme expert Thomas R. Ward of the University of Basel. By identifying the right protein on which to base their design, Hartwig and coworkers “have laid their hands on a gold mine,” Ward says. “The work brings metalloprotein design and evolution into the next league—to a ball park that should interest industry.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter