Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Anion dimer captured within cyanostar macrocycles
Study provides first direct evidence for hydrogen-bonded anion pairs
by Jyllian Kemsley
October 31, 2016
| A version of this story appeared in
Volume 94, Issue 43
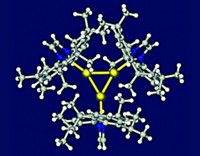
Opposite charges attract and like charges repel, Coulomb’s law says. But anions can pair up and stabilize each other, according to research led by Amar H. Flood of Indiana University, Bloomington (Angew. Chem. Int. Ed. 2016, DOI: 10.1002/anie.201608118). Although indirect evidence had previously pointed to the existence of dihydrogen phosphate dimers, direct evidence was lacking. Combining macrocyclic cyanostars and bisulfate, HSO4–, Flood and coworkers expected to create a complex with one bisulfate anion sandwiched in the center of two cyanostars, as already documented for perchlorate. Instead, they observed a 2:2:2 complex incorporating a pair of bisulfate anions hydrogen-bonded to each other, nestled in the centers of two stacked cyanostars and capped by tetrabutylammonium cations. The anion dimer is stabilized by hydrogen-bonding interactions to the cyanostar’s cyanostilbene-based C–H groups. The complex persists in both crystalline form and in solution, as documented by X-ray crystallography and 1H NMR. The system could point to new ways to recognize and sequester ions, the researchers say.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter