Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Gene therapy is ready for its comeback
Clinical successes, focused start-ups, and pharma’s attention push genetic remedies closer to market
by Ann M. Thayer
November 14, 2016
| A version of this story appeared in
Volume 94, Issue 45

Attempts to treat disease by inserting DNA into patients’ cells all but ended in 1999 after the death of an 18-year-old from a severe immune response to the virus used to deliver a corrective gene. Companies and investors left the field in droves.
Research retreated mainly to academic medical centers. Scientists—including the University of Pennsylvania’s James M. Wilson, who had been involved in the tragic trial—sought viral vectors that could be both safe and effective. Instead of the adenoviruses that had been used in the trial, they experimented with adeno-associated viruses (AAVs), smaller viruses that infect people but are not pathogenic and show little immunogenicity.
Eventually, results began to emerge from tests of AAVs used for in vivo gene delivery, as well as retro- and lentiviral vectors for ex vivo therapies. In 2003, China approved the cancer gene therapy Gendicine, which uses an adenovirus. By the late 2000s, gene therapy clinical trials were well under way, approaching more than 100 per year.
In 2012, the Dutch firm UniQure received European approval for Glybera (alipogene tiparvovec). The AAV-based therapy treats an ultrarare condition called lipoprotein lipase deficiency. In 2015, UniQure’s partner Chiesi Farmaceutici treated the first, and so far only, patient, reportedly at a price of more than $1 million. Although not a commercial success, Glybera proved that a regulatory path exists.
Along with positive events came the interest of investors and large drug companies. Start-ups emerged to develop better AAV vectors and therapies. These typically aim to restore function of a single missing or mutated gene in diseases such as cystic fibrosis and hemophilia, as well as some neurodegenerative, metabolic, and retinal disorders. As it hits technical and regulatory milestones, gene therapy is poised to become a fully realized way to treat disease.
Thus far, demonstrating where gene therapy can and can’t work has been on a “tissue by tissue” basis, says Ken Mills, chief executive officer and a founder of Regenxbio. Most clinical trials are still early stage.
“In 2016 and especially 2017, we are coming into the age of significant clinical demonstrations,” Mills says. “Meaningful data are going to both de-risk the platform further and then translate into an even more significant catalyst for real commercial interest.”
Since early 2014, nine start-ups have raised nearly $900 million through initial public offerings of stock. And major drug companies have allied with many of them in deals worth up to $1 billion in potential milestone fees and equity investments. For example, Bristol-Myers Squibb is working with UniQure on cardiovascular disease, and Sanofi has joined with Voyager Therapeutics for neurological conditions.
Meanwhile, Biogen has committed up to $2 billion to Penn in the ophthalmic area and separately licensed AAV vectors from Regenxbio. Biogen also has a $1 billion ophthalmic deal with Applied Genetic Technologies Corp. (AGTC).
Hemophilia, for which durable gene therapy results have been reported (N. Engl. J. Med. 2014, DOI: 10.1056/NEJMoa1407309), is also attracting big pharma. Pfizer is working with Spark Therapeutics, Shire with Regenxbio, and Bayer with Dimension Therapeutics, a 2013 Regenxbio spin-off.
Regenxbio itself was formed in 2009 through the efforts of Penn, advisory firm FoxKiser, and GlaxoSmithKline, which had spent nearly $40 million over eight years to support Wilson’s work. Regenxbio says it has rights to more than 100 novel AAVs.
Compared with earlier generations of AAV vectors, Regenxbio’s newer ones address key attributes needed for gene therapy to succeed, the firm says. These include increased, long-term gene expression; broad tissue selectivity; decreased immune response; and improved manufacturability.
Internally and through licensees, Regenxbio’s vectors are being used in about 30 development candidates, Mills points out. And according to a database managed by the, the proportion of clinical trials using AAV vectors has been increasing, reaching about 25% in 2016.
Cancer accounts for 64% of all gene therapy trials and is the target of many ex vivo approaches, including chimeric antigen receptor T cells. At 10%, the next biggest group is monogenic, often rare, diseases.
Regenxbio’s internal rare disease pipeline includes a candidate for treating homozygous familial hypercholesterolemia that uses an AAV vector to deliver the human low-density lipoprotein receptor gene to liver cells. It is also targeting Mucopolysaccharidosis type I (MPS I), or Hurler syndrome, by delivering the α-L-iduronidase gene to the central nervous system. Similarly, the company has a therapy for MPS II, known as Hunter syndrome, that conveys the human iduronate-2-sulfatase gene.

But ophthalmic disease is one of the most advanced areas because the eye is a practical gene therapy target. “It is a confined and immunoprivileged space,” explains Katherine High, chief scientific officer and a cofounder of Spark Therapeutics. Because of the eye’s compact size, very little material is needed, which means that manufacturing requirements are modest.
Spark recently reported positive Phase III results for voretigene neparvovec, an AAV-based therapy that treats an inherited retinal disease caused by mutations in the RPE65 gene. Vision was restored in 93% of patients, and data collected so far show an effect in humans lasting for three years. A rolling New Drug Application is underway with the Food & Drug Administration, and approval could come in early 2017.
Expectations are that Spark’s RPE65 therapy will be the first gene therapy approved by FDA to treat a genetic disease. Although there are only about 3,500 potential patients, analysts are estimating peak annual sales of at least $400 million for what is expected to be a very high priced but potentially one-time injection.
The fact that Spark has enough regulatory-compliant manufacturing capacity is actually a consequence of the retrenchment that occurred after 1999, High says. At the time, High was leading the Center for Cellular & Molecular Therapeutics, part of the Children’s Hospital of Philadelphia, which was working to establish proof of concept for gene therapy in the eye and liver. To keep work going after a biotech partner exited gene therapy, it built its own facility.
As the center’s retinal disease therapy neared Phase III studies, “we started basically getting cold calls from pharma companies looking to partner with us,” High says. “But the reality was that most of them had very little experience in gene therapy.” Instead, with in-house research and clinical, regulatory, and manufacturing expertise in place, the hospital spun off Spark in 2013.
The ability to produce commercial-scale materials is “one of the big areas missing in the field,” says Greg LaRosa, chief scientific officer of Pfizer’s rare disease unit. Manufacturing capabilities contributed to Pfizer’s interest in acquiring Bamboo Therapeutics in August. Earlier in the year, Bamboo had purchased a 1,000-m2 facility from the University of North Carolina, Chapel Hill.
With Bamboo, Pfizer also gained preclinical and clinical AAV-based gene therapies in the central nervous system and neuromuscular areas, including one targeting Duchenne muscular dystrophy that is expected to enter clinical trials in 2017 (see page 36). “These indications are going to require fairly large doses because they are big organ systems,” LaRosa says.
Before making acquisitions and setting up collaborations, Pfizer invested internally. In 2014, it created the Genetic Medicines Institute within its rare disease unit. To run it, the company recruited AAV expert Michael Linden, a former director of University College London’s Gene Therapy Consortium.
“About a year and a half ago, we felt that AAV-mediated in vivo gene therapy as a technology had come a long way from the early days,” LaRosa says. Pfizer considered it a way to bring new treatment opportunities to its rare disease efforts. Its institute focuses on AAV vector and process development.
“We have found that the intellectual property space around AAV is pretty complex,” LaRosa says. “And we are not sure where it is all going to shake out in the end, so we want to have capability in house to develop our own novel AAV vectors with the sort of tissue targeting that we need.”
Gene green
Big pharma has been investing in developers of AAV-based gene therapies.
| Company | Partner | Targets | Year | Up-front Fees ($ Millions) |
|---|---|---|---|---|
| Allergan | RetroSense Therapeuticsa | Eye | 2016 | $60 |
| Bayer | Dimension Therapeutics | Hemophilia | 2014 | 20 |
| Biogen | Applied Genetic Technologies Corp. | Eye | 2016 | 124 |
| University of Pennsylvania | Eye, skeletal muscle, CNS | 2016 | 83 | |
| Bristol-Myers Squibb | UniQure | Heart | 2015 | 100 |
| Pfizer | Bamboo Therapeutics a | CNS | 2016 | 150 |
| Spark Therapeutics | Hemophilia | 2014 | 20 | |
| 4D Molecular Therapeutics | Heart | 2016 | na | |
| King's College London | Viral vectors | 2016 | na | |
| University of Iowa | Cystic fibrosis | 2016 | na | |
| Roche | 4D Molecular Therapeutics | na | 2015 | na |
| Sanofi | Voyager Therapeutics | CNS | 2015 | 100 |
a Acquisition. CNS = central nervous system. na = not available.
In January, Pfizer invested in and began working with 4D Molecular Therapeutics, which has also partnered with Roche, UniQure, and AGTC. The three-year-old firm discovers and develops AAV vectors and gene therapies using technology from the lab of cofounder and University of California, Berkeley, professor David Schaffer.
Although gene therapies are proving their worth, many “are special cases where the delivery burden is a bit lower and, as a result, a natural version of the virus when administered through pretty invasive routes has been just good enough to get that efficacy,” says Schaffer, who is also 4D’s acting chief scientific officer. “To begin to build on that success and that momentum and to begin to extend it to tougher disease targets, we quite simply need better viruses.”
AAVs have shortcomings as gene delivery vehicles, including limited capabilities for targeting specific tissues and the cells within them and then penetrating those cells. Diminishing returns at each step mean large quantities may be needed for a therapy to work. “We think we can get better efficacy,” even for disease targets for which there are already positive results, Schaffer says.
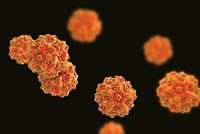
An AAV’s protein shell, or capsid, is what carries the therapeutic cargo from administration until it arrives in the nucleus of the target cell. About 80 to 85% of the structure is the same among various AAVs. Modest-sized protrusions on the surface are what dictate how an AAV interacts with cell and tissue receptors, moves across membranes, and protects its DNA cargo.
To expand the structural diversity and properties, Wilson isolated and generated in vitro hundreds of AAVs. To better understand why some vectors perform better than others, his lab launched a program this summer that combines molecular, structural, and cell biology with bioinformatics.
Meanwhile, 4D addresses the need for diversity through directed evolution. “We have genetically diversified those capsid proteins and created 100 million different versions within our large library,” Schaffer says. “We can select from that library to isolate the optimal virus, rather than being at the mercy of one of the 10 natural viruses that people are using in clinical trials these days.”
Diversity can also be important in choosing an AAV to use. Although AAVs are not human pathogens, most people have been exposed to them and have developed antibodies. A preexisting immunity can render a gene therapy ineffective and exclude an individual from treatment.
To circumvent this problem, 4D has “created new engineered versions of AAVs that are largely resistant to preexisting antibodies in the human population,” Schaffer says. “We believe that we are going to be able to treat a larger fraction of the human population that comes into the clinic.”
However, even if people haven’t previously been exposed to a particular AAV, in the course of therapy they may raise new antibodies, which would prevent them from getting subsequent doses. To avoid this, many gene therapy protocols use transient immunosuppression to knock down immune responses.
But gene therapy developers strive to do better. “Potentially, a single administration is going to be sufficient to get years or possibly even greater than a decade of therapeutic efficacy,” Schaffer says.
Even if the vector’s cell-targeting ability is optimized, AAV gene therapy is still not suitable for all diseases. The AAVs employed today work best in nondividing cells, such as those in the eye, because the genetic material is expressed but not integrated into the chromosomes. In dividing cells, nonintegrated code isn’t replicated and will be diluted. Expression is eventually lost.
On the plus side, a nonintegrating vector avoids the possibility of random chromosomal insertions and potentially harmful mutagenesis in the body. For diseases that involve rapidly dividing cells, such as cancer, integrative lentiviral and other vectors are often used ex vivo in isolated target cells.
Advertisement
Another drawback can be AAVs’ carrying capacity. At best, just over 5,000 base pairs of DNA can be crammed in, which limits the size of the gene that can be transported. This still allows for encoding up to a few average-sized human proteins, but very large proteins and complex or multigene diseases might be harder to address.
One solution may be going with entirely different payloads. Among these is the hot genome-editing tool CRISPR/Cas9, for which AAV vectors could emerge as the in vivo ride for a high-profile passenger. “CRISPR/Cas9 is an incredibly powerful cargo, but it needs a vehicle to be delivered to the right tissue,” Schaffer says.
Thanks to AAV, gene therapy is coming of age. The combination of AAV and CRISPR has the potential to carry gene therapy beyond the realm of simple gene addition into the editing, repair, or replacement of an even broader range of disease-causing genes.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter