Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Development
Lilly Alzheimer’s drug fails
The firm will not seek regulatory approval for solanezumab
by Lisa M. Jarvis
November 23, 2016
| A version of this story appeared in
Volume 94, Issue 47

In yet another blow to the amyloid hypothesis, Eli Lilly & Co. said today that its Alzheimer’s treatment solanezumab failed in a Phase III study. Shares of Lilly were down as much as 15% on the news.
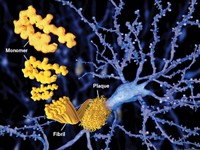
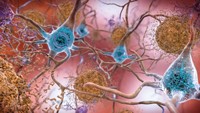
For more than a decade, Alzheimer’s drug developers have focused their efforts on trying to prevent or remove the amyloid plaques coating the brains of people with the disease. Solanezumab, which binds to soluble, monomeric forms of amyloid-β, had already tanked in two Phase III studies. But Lilly decided to push on with a third trial based on signs that people with early stage Alzheimer’s benefited from the drug.
In this latest study, the drug failed to slow down the cognitive decline seen in the disease. Lilly said it would no longer file for regulatory approval for the drug, which, if approved, would easily have enjoyed multi-billion-dollar sales.
“This result will no doubt cast a shadow over Lilly’s Alzheimer’s disease pipeline portfolio, which is heavily based on the beta-amyloid hypothesis,” Leerink stock analyst Seamus Fernandez told clients. The news is also hitting Biogen, which has aggressively moved ahead late-stage studies of aducanumab, an antibody targeting aggregated amyloid-β. Biogen’s stock was initially down more than 8% this morning.
The solanezumab failure adds to a graveyard of Alzheimer’s disease treatments. Indeed, Lilly already had plots there: Solanezumab is the third Alzheimer’s drug the firm has dropped from its neuroscience pipeline. In 2010, the company pulled the plug on semagacestat, a small molecule that blocks γ-secretase, after two failed Phase III studies. Three years later, Lilly ended development of LY2886721, a BACE inhibitor that had made it to Phase II.
Many are wondering whether solanezumab’s failure should be seen as the final word on the amyloid hypothesis. Sam Gandy, an amyloid expert at Mount Sinai’s Alzheimer’s Disease Research Center who for months had predicted the failure, noted the interventions were likely happening too late in the disease. “By the time an amyloid scan is positive,” he says, microscopic clumps of amyloid- β “may have been there for decades.”
Other Alzheimer’s experts say solanezumab itself may be the problem. “It is not truly surprising, as sola was always known to be a ‘weak’ antibody in terms of its ability to mobilize amyloid from the brain,” says Dennis Selkoe, a Harvard neurologist who was among the first to put forward the amyloid hypothesis in the early 1990s.
Selkoe argues that Biogen’s aducanumab, “which appears to be much more effective at mobilizing amyloid- β,” and a small molecule from Merck still hold promise.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter