Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Enzyme’s structure helped elucidate RNAi’s mechanism
Study confirmed how RNA-cleaving “Dicer” enzyme measures and then snips its substrates, advancing our understanding of gene silencing
by Stu Borman
December 18, 2016
| A version of this story appeared in
Volume 94, Issue 49

Ten years ago, researchers obtained the first crystal structure of Dicer, a key enzyme in a biological process called RNA interference (RNAi). In RNAi, a process also known as gene silencing, a short RNA binds to mRNA, preventing mRNA’s sequence from being transcribed into a protein.

The determination of Dicer’s structure by Jennifer A. Doudna of the University of California, Berkeley, and coworkers helped confirm how the enzyme processes RNA and thus advanced RNAi technology (Science 2006, DOI: 10.1126/science.1121638). More recently, Doudna and coworkers also helped discover CRISPR-Cas9, a technology with broader gene-editing applications.
RNAi was discovered about 20 years ago by Andrew Z. Fire and Craig C. Mello when they were researchers at the Carnegie Institution of Washington. Fire and Mello won a 2006 Nobel Prize for this work because it revealed RNAi to be a previously unknown mechanism for gene regulation and opened up the potential for a new class of antisense oligonucleotide therapeutics.
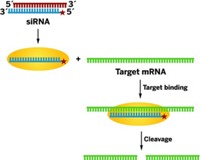
Dicer kick-starts the RNAi process by cleaving either a type of hairpin RNA, called precursor miRNA, or double-stranded RNA. The cleavage products—microRNA (miRNA) and small interfering RNA (siRNA), respectively—are approximately 22-nucleotide double-stranded RNA fragments. A protein named Argonaute 2 then takes up these fragments, releases one strand (the passenger) of each fragment, and uses the other strand (the guide) to bind target mRNA. Finally, Argonaute 2 cleaves the target mRNA, silencing the gene it encodes.
The Doudna group’s Dicer structure showed that a formation called the “handle,” located between the enzyme’s PAZ and RNase III domains, is about the same length as 25 RNA nucleotides. This helped confirm that Dicer acts as a molecular ruler, cleaving its RNA substrates into approximately handle-sized pieces during the first step of RNAi. The study also helped confirm that two magnesium ions in each of the enzyme’s two RNase III domains play essential roles in its mechanism of action.
Since its discovery, RNAi has been used as a research tool to study biological processes such as cancer and cell differentiation. It is also being developed commercially today as a means to block the production of disease-related proteins and potentially as a means to control insect pests in agriculture. But the Dicer structure per se didn’t help lead to RNAi therapeutics, which “bypass Dicer by delivering RNA that is already cut like the Dicer product,” explains Judy Lieberman of Boston Children’s Hospital, whose research interests include RNAi.
A number of these Dicer-bypassing therapeutics—siRNAs—are now in the clinical trials pipeline for various diseases. But RNAi agents have had cell-access problems. “The real obstacle is getting them across the cell membrane” and released inside target cells, Lieberman says. Chemically conjugating siRNAs with a sugar recognized by a liver cell receptor or encapsulating siRNAs into lipid nanoparticles have largely solved these problems for the liver, but not other tissues in the body, she says.
So no RNAi agents have yet been approved for clinical use. Nevertheless, Lieberman says, “I remain cautiously optimistic that they will be a new class of very powerful drugs. We will know more in the coming year.”
C&EN's YEAR IN REVIEW
Top Headlines of 2016
- Hawaii explosion cost a researcher an arm
- TSCA reform crossed the finish line
- U.S. elected Trump as president
- The periodic table got four new elements
- Post Dow-DuPont, chemical deal-making waned as 2016 advanced
- Labs made advances in Zika research
- Four ag giants to rule them all
- 2016 Nobel Prize in Chemistry at a glance
- Brexit bomb exploded
- Focus returned to Iran's chemical industry
- Overtime pay limit doubles under Obama administration
- The CRISPR craze continued
- Perfluorinated compounds got increased scrutiny
- From the lab to the market
- Paris Agreement to curb climate change took off
- World chemical production at a glance
- Flint's water woes lingered
- As China's economy slowed, chemical makers adjusted
- Mostafa A. El-Sayed won the 2016 Priestley Medal
- ACS members on average fared better than young graduates in employment surveys
- ACS invests internationally
- Spun-off firms found their footing in 2016
- Remembering the Nobel laureates we lost in 2016
- ACS proposed chemistry preprint server
- Berkeley College of Chemistry avoided reorganization
- Fight against opioid epidemic continued in 2016
- Noble gas shortages averted, for now
- The shale gas boom by the numbers
- Crop protection products in the crosshairs
- ACS launched new journals
- Website search terms of the year
- Biobased materials hit the big time
- No better deal emerged for Mossville, La.
- U.S. prepares for national food labeling standard
Top Research of 2016
- Mini factory made drugs on demand
- World's first PET-munching microbe discovered
- Liquid metals went to work
- Methylene activation reached new heights
- Biological structures of the year
- Wearable sensors were 'the' fashion accessories of 2016
- Scientists beefed up the antibiotic arsenal
- An enzymatic route to carbon-silicon bonds
- Single-atom catalysts gained a toehold
Revisiting Research of 2006


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter