Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Another Way To Functionalize Alkynes
Organic Synthesis: tert-Butyl nitrite offers a metal-free approach to alkyne bond breaking and aryl nitrile making
by Stephen K. Ritter
February 26, 2016
| A version of this story appeared in
Volume 94, Issue 8

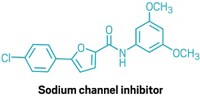
Alkynes are versatile reagents that lend themselves to an array of functionalizations—oxidation, cross-coupling, cycloaddition, and metathesis reactions, to name a few. In seeking out additional examples, Uttam Dutta and Debabrata Maiti of Indian Institute of Technology Bombay (IITB) and David W. Lupton of Monash University have teamed up to develop the first example of metal-free nitrogenation of terminal alkynes to produce aryl nitriles (Org. Lett. 2016, DOI: 10.1021/acs.orglett.6b00147). The team, part of the IITB-Monash Research Academy, developed the reaction based on the Maiti group’s earlier successes in using tert-butyl nitrite for the C–H functionalization of alkenes and alkynes to make nitroolefins and α-nitroketones, respectively. When the researchers came across literature reports of using trimethylsilylazide as a reagent for converting alkynes to nitriles, it occurred to them that tert-butyl nitrite could provide a safer and cost-effective alternative to azides. The Maiti-Lupton team coupled tert-butyl nitrite with 2-picoline-N-oxide as an oxidant to make more than three dozen aryl and heteroaryl nitriles up to gram scale (one shown), including natural product derivatives such as converting the alkynyl ester of vitamin E to a nitrile.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter