Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Cyanotriflation gives versatile acrylonitriles
Cyanoiodonium triflate reagent with an iron catalyst offers first method for direct vicinal cyano-triflate alkyne additions
by Stephen K. Ritter
February 29, 2016
| A version of this story appeared in
Volume 94, Issue 9

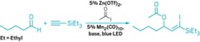
Chemists in Germany have added a new dimension to preparing acrylonitriles with the first method for adding both a cyano group and a triflate group to an alkyne (J. Am. Chem. Soc. 2016, DOI: 10.1021/jacs.6b00869). Xi Wang and Armido Studer of the University of Münster took advantage of a known aryl(cyano)iodonium triflate reagent that other groups have used to transfer the cyano group or the triflate group. But until now, no one had devised a reaction system to add both groups to another molecule at once, in this case a range of alkynes and diynes. The Münster team’s syn addition proceeds with regio- and stereochemical control at up to gram scale via a radical mechanism mediated by an iron phenanthroline catalyst, leading to a host of triflate-modified acrylonitriles. The researchers found that the triflate provides a handle for further functionalizing the acrylonitriles via palladium-catalyzed cross-coupling reactions (example shown) to make even more complex molecules for pharmaceutical and materials applications.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter