Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Reaction Dynamics
Happy Accident Leads To Faster Synthesis
Protein Chemistry: Power outage reveals that oxime ligation speeds up below freezing
by Katharine Sanderson
January 8, 2016
When a power outage hit the labs of biochemist Tilman M. Hackeng of the University of Maastricht, his colleague Stijn M. Agten rushed to remove some samples from their ultra-performance liquid chromatography-tandem mass spectrometry machine. Agten was monitoring the progress of an oxime ligation reaction, and to give himself time to reboot the machine, he froze his samples to -20 ˚C, expecting to slow the reaction rates and allow him to salvage something from his efforts.

But when the sample thawed, Agten was shocked to see that his reaction was almost complete (Bioconjugate Chem. 2015, DOI: 10.1021/acs.bioconjchem.5b00611). “Normal oxime ligations take days to get acceptable yields of partial completion,” Hackeng says.
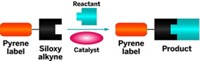
In oxime ligations, an aldehyde or ketone reacts with an aminooxy group on another molecule to form an oxime bond. Hackeng and his colleagues, who are investigating oxime ligations as a way to label proteins, were testing how temperature, concentration, and catalysts affect the rate of oxime formation when they made their discovery.
The team tried to shorten the freezing time by using lower temperatures: -80 and -196 ˚C. But the hour-long freeze at -20 ˚C they uncovered by accident sped up the reaction most effectively. This is probably because slow-growing ice crystals push out the reactants and concentrate them in the liquid phase, Hackeng says.
They also discovered that by freezing, they could run the reaction without a catalyst at a neutral pH and still get better results than adding aniline as a catalyst and reacting at low pH, as is typically done for oxime ligations at warmer temperatures. They then tested the technique by labeling a model protein system. After three freezing and thawing cycles, the reaction was 91% complete—a stark improvement on the usual method, which left the reaction only 10% complete after 48 hours.
Hackeng says that the notion of accelerating a reaction by freezing is “completely counterintuitive,” though scouring the literature brought up a few other examples.
Meanwhile, the serendipitous finding has had great practical effect: “We use it every day,” he says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter