Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Diamonds expose deep-Earth chemistry
Inclusions in precious gems incorporate vestiges of metallic liquid from a reducing environment
by Jyllian Kemsley
January 2, 2017
| A version of this story appeared in
Volume 95, Issue 1
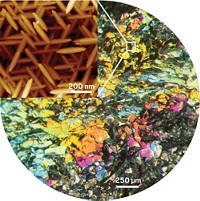
Some diamonds—including large, exceptional gems similar to the Cullinan, Constellation, and Koh-i-Noor—carry with them remnants of the metallic liquid from which they formed deep in Earth’s mantle, according to new research (Science 2016, DOI: 10.1126/science.aal1303). The findings confirm predictions from theoretical and laboratory studies that Earth’s deep mantle could have highly reducing regions capable of producing iron alloys, but for which researchers lacked direct evidence. By using Raman spectroscopy, X-ray diffraction, and scanning electron microscopy, a team led by Evan M. Smith of the Gemological Institute of America studied a particular class of diamonds that are large, inclusion-poor, relatively pure, and irregularly shaped, and that show evidence of partial dissolution as they rose to Earth’s surface. The team found that diamonds of this particular class contain inclusions of a metallic mix of iron, nickel, carbon, and sulfur surrounded by a thin fluid jacket of methane and hydrogen. That combination of materials indicates that the diamonds formed from a metallic liquid in a reducing environment, the team explains.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter