Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Quantum effect could explain how chiral molecules interact
Electron spin polarization promotes recognition between molecules of similar chirality
by Jyllian Kemsley
March 9, 2017
| A version of this story appeared in
Volume 95, Issue 11

Biomolecules from small amino acids to large DNA helices are chiral, and how they interact depends on their chirality. A newly identified quantum effect could help explain how biomolecules’ chirality persists.
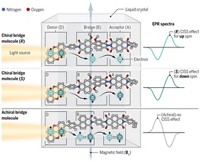
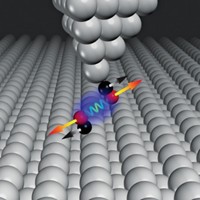
When two molecules interact, their electron clouds reorganize. In chiral molecules, that reorganization is accompanied by electron spin polarization that enables molecules of the same chirality to interact more strongly than molecules of opposite chirality, reports a research team led by Ron Naaman and Jan M. L. Martin of the Weizmann Institute of Science and David H. Waldeck of the University of Pittsburgh (Proc. Natl. Acad. Sci. USA 2017, DOI: 10.1073/pnas.1611467114).
“The mechanism that they have demonstrated is different from any that was previously reported,” comments David N. Beratan of Duke University. “If the idea holds up, it could entirely change the way we think about molecular recognition in biological and organic chemistry.”
In the new work, Naaman and colleagues combined experimental studies of helical oligopeptides with computational analysis. They found that the electron spin polarization induced by molecular interactions constrains the symmetry of the wave functions involved in the interactions. The symmetry constraints in turn lead to energy differences when molecules of the same chirality interact compared with molecules of opposite chirality, favoring homochiral interactions.
“It’s a real quantum mechanical property that can’t be represented in classical-physics-based models,” Naaman emphasizes.
The effect is short-range and arises only when electrostatic and other noncovalent interactions bring molecules together, making it more likely to influence enantiospecific recognition in a crowded cellular environment than in dilute solutions. It is also additive and becomes more significant when many chiral functional groups interact across the surfaces of large biomolecules.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter