Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Targeting one protein to treat two neurodegenerative diseases
Disrupting production of ataxin-2 with antisense technology improves symptoms of ALS and spinocerebellar ataxia type 2 in mice
by Michael Torrice
April 12, 2017
| A version of this story appeared in
Volume 95, Issue 16
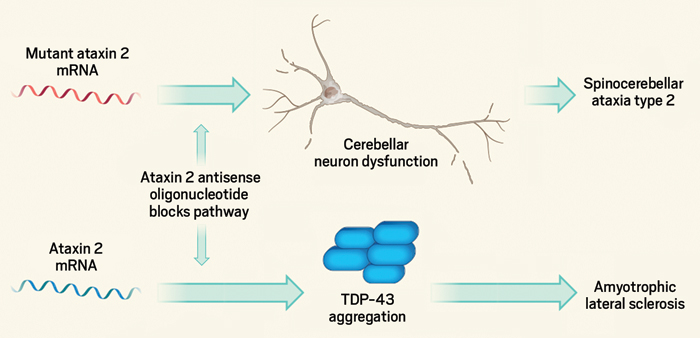
Reducing the production of a certain protein in the brain could lead to treatments for two different neurodegenerative diseases—spinocerebellar ataxia type 2 (SCA2) and amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease—according to a pair of studies published in Nature.
“I’m very excited about the papers,” says Beverly L. Davidson of Children’s Hospital of Philadelphia, who was not involved in the work. “They’re not cures but they show positive effects. And for these two disorders, that’s a good thing.”
The protein that the two overlapping research teams target is ataxin-2. The exact function of ataxin-2 in the brain is not fully understood, but people with SCA2 have many extra copies of a three-nucleotide repeat, CAG, in their version of the ataxin-2 gene. Expression of the resulting mutant protein causes the loss of neurons mainly in the cerebellum, leading to difficulty in speech and in coordinating movements of the limbs and eyes.
To reduce levels of this mutant protein, Stefan M. Pulst of the University of Utah and colleagues employed antisense oligonucleotide (ASO) technology to target and destroy messenger RNA transcribed from the ataxin-2 gene in the brain. ASOs are short polymers of chemically modified nucleic acids that bind to certain sequences in a specific gene’s mRNA. Cellular machinery recognizes the resulting nucleic acid complex and degrades the mRNA. The ASO is left untouched and can go bind another transcript and trigger the process again.
Pulst’s team developed an ataxin-2-targeting ASO with the help of scientists at Ionis Pharmaceuticals, which makes ASOs to treat nervous system disorders. When injected into the brains of mice, the ASO reduced expression of ataxin-2 by as much as 75%. The ASO slowed the loss of motor function in two types of mice that develop SCA2-like symptoms (Nature 2017, DOI: 10.1038/nature22044).
“This is a big step forward,” says Teepu Siddique of Northwestern University. Despite not fully understanding the function of ataxin-2 or how the expanded CAG repeats cause disease, the researchers could use ASO technology to target the protein to yield positive effects in mouse experiments.
In the second paper, a team led by Aaron D. Gitler of Stanford University tested an ataxin-2-targeting ASO in mice with an ALS-like condition.
ALS patients suffer from a progressive weakening of muscles that is eventually fatal. Unlike with SCA2, many cases of ALS aren’t genetic. But Gitler previously found that ataxin-2 interacts with TDP-43, a protein that forms toxic aggregates found in many ALS patients’ brains. Reducing levels of ataxin-2 decreases the toxicity of these aggregates in yeast and fruit flies.
So in the new study, Gitler and his colleagues, including Pulst and scientists at Ionis, tested whether knocking down ataxin-2 expression had an effect on ALS-like symptoms in mice engineered to overexpress TDP-43. These animals form toxic TDP-43 aggregates in their brains and die within 30 or so days. An injection of an ataxin-2-targeting ASO extended the median lifespan of these mice by 35%, with a couple mice living for 120 days (Nature 2017, DOI: 10.1038/nature22038).
Pulst considers both studies proofs of principle that ASO technology could lead to feasible treatments for SCA2 and ALS. In general, he thinks ASO technology is well suited for targeting neurodegenerative disorders given that scientists don’t fully understand the mechanisms behind the diseases. “I call this a pathway-agnostic approach,” Pulst says. “In the end, we don’t really need to understand what a protein does as long as we can down-regulate it and we can show that the down-regulation itself isn’t harmful.”
Also, Pulst points out that the Food & Drug Administration has already approved an ASO treatment for one nervous system disorder—spinal muscular atrophy—and a few others are in clinical trials.
Davidson thinks this track record means that ataxin-2-targeting ASOs have good translation potential. “There is enough safety data in humans for ASOs, so these could move fairly quickly into human clinical studies,” she says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter