Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chemists can now be picky about organosulfur compounds
A new strategy enables direct selective C–H functionalization of a single sulfur component from mixtures found in petroleum refinery streams
by Stephen K. Ritter
April 24, 2017
| A version of this story appeared in
Volume 95, Issue 17

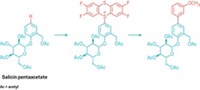
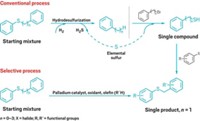
When hearing someone describe the typical preparation of organosulfur compounds, it’s clear the approach is not a very sustainable one. Mixtures of sulfur compounds naturally found in crude oil are first treated with hydrogen in a catalytic refinery process to remove the sulfur—the goal is to pull out as much sulfur as possible to create cleaner transportation fuels. The hydrogen sulfide that forms in this hydrodesulfurization step is converted to elemental sulfur. Chemists then pair this sulfur with selected reactants to reconstruct desired organosulfur compounds one product at a time. Overall, the energy-intensive, multistep process is costly and generates a significant amount of waste. A new strategy reported by Valentine P. Ananikov of the Russian Academy of Sciences’ Zelinsky Institute of Organic Chemistry and coworkers enables unprecedented direct C–H functionalization and separation of only one sulfur component at a time from mixtures of organosulfur compounds (ACS Omega 2017, DOI: 10.1021/acsomega.7b00137). The Russian researchers use palladium acetate along with oxygen and silver trifluoroacetate as a combination oxidant to couple an olefin, such as ethyl acrylate, to an aryl or heteroaryl sulfur compound. The reaction’s selectivity centers on the sulfur acting as a directing group to guide the C–H activation and alkenylation. Benzyl compounds provide the most favorable geometry for palladium binding, followed by compounds with longer alkyl chains, which leads to exclusive functionalization of one component at a time in a mixture.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter