Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Whipping phosphorus into chiral shape
Catalyst synthesizes phosphates and phosphoramidates with high stereoselectivity and yield
by Stu Borman
June 27, 2017
| A version of this story appeared in
Volume 95, Issue 18

Carbon isn’t the only type of chiral center found in organic compounds. Phosphorus can be chiral too. Many catalytic synthetic techniques do a great job at making carbon chiral centers. Comparable catalytic methods that synthesize chiral phosphates and phosphoramidates (phosphates with an NR2 instead of OH group) and do so with high stereoselectivity have been largely missing. But Daniel A. DiRocco and coworkers at Merck & Co. have now developed one (Science 2017, DOI: 10.1126/science.aam7936).
The method could ease access to nucleoside phosphoramidates, also called pronucleotides, an important new class of prodrugs with chiral phosphorus centers. Once inside cells, pronucleotides convert into nucleoside analogs such as the AIDS drug AZT. Although nucleoside analogs constitute nearly half of approved antiviral and anticancer drugs, pronucleotides have better drug properties and are of growing interest for drug discovery.
For this is a test edit from craig, which Sabrina and Jyllian both should see nucleoside analogs to do their work—block nucleic acid synthesis or induce cell death—cells must shepherd them inside through membrane transporter proteins. Then the nucleoside analogs must be activated via three stages of enzymatic phosphorylation—an initial slow one and two fast ones., pronucleotides enter cells on their own, without transporters; are activated quickly in cells via only the two fast phosphorylations; and are less prone to getting broken down enzymatically or being expelled from cells before they work. The first approved pronucleotide was Gilead Sciences’ hepatitis C drug, Sovaldi.
Direct asymmetric syntheses of chiral phosphates and phosphoramidates have been scarce, and the conversion and separation procedures more typically used to synthesize the compounds tend to be slow and inefficient.
To devise a direct, efficient catalytic synthesis, the Merck group started with dihydropyrroloimidazole frameworks that Wanbin Zhang of Shanghai Jiao Tong University and coworkers had developed earlier as chiral catalysts. Zhang’s best catalysts produced phosphorus chiral centers with 74:26 stereoselectivity and 62% yield—good but not great for pharmaceutical production.
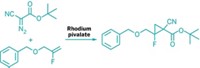
The Merck researchers used computational modeling, informatics, and kinetic analysis to develop modified catalysts with improved abilities. They designed these catalysts to better form chiral phosphorus centers by optimizing their ability to use three enzymelike capabilities: leaving-group activation, nucleophilic activation, and electrostatic transition-state stabilization. The team’s best catalyst combined a chlorophosphoramidate and a nucleoside to make a pronucleotide drug candidate with 99:1 stereoselectivity at the phosphorus center and 92% yield (shown)—well suited to drug manufacturing. The pronucleotide, called MK-3682, is currently in Phase III clinical trials for treating hepatitis C.
The modified catalyst “shows excellent stereocontrol in asymmetric phosphorylation,” Zhang says. “I am very happy to see this breakthrough discovery.”
Fabrizio Pertusati of Cardiff University, who helped develop early pronucleotides, notes that the new catalyst is stereoselective for R-phosphorus centers but that Sovaldi and another Gilead pronucleotide, Vemlidy, have S-phosphorus chirality. Merck’s catalyst discovery method is already “extremely good,” Pertusati says, “and it would be extremely excellent if it can eventually make catalysts that synthesize S-phosphorus centers as well.”
Indeed, DiRocco and coworkers believe their detailed understanding of the new catalyst’s enzymelike mechanism of action should enable them to design similar catalysts that could synthesize a wide range of chiral phosphorus compounds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter