Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Making chiral zeolites in bulk
Enantio-enriched molecular sieves separate chiral molecules and mediate chiral reactions
by Mitch Jacoby
May 4, 2017
| A version of this story appeared in
Volume 95, Issue 19

Composed of a network of molecule-sized channels and pores, zeolites have been used for decades in catalysis, separations, ion exchange, and adsorption. Researchers would like to make chiral versions of these molecular sieves for separating mixtures of stereoisomers and carrying out chiral reactions. Yet making bulk samples of chiral zeolite crystals that are made entirely of one enantiomer has remained an elusive goal.
A team of researchers led by Mark E. Davis of California Institute of Technology has now synthesized enantio-enriched batches of germanosilicate molecular sieves exhibiting R and S chirality. The team has used these materials to separate chiral molecules and as heterogeneous catalysts to mediate chiral reactions (Proc. Natl. Acad. Sci. USA 2017, DOI: 10.1073/pnas.1704638114).
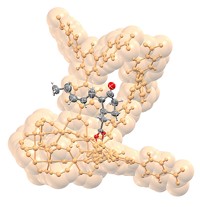
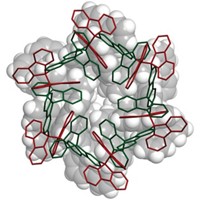
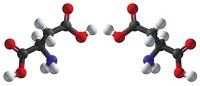
To make the materials, Davis and coworkers used computational methods to design and evaluate organic structure-directing agents (OSDAs), which are used to guide the three-dimensional assembly of zeolite components. They synthesized enantiopure samples of a chosen OSDA, a bisimidazolium salt, and reacted each of its enantiomers separately with silicon- and germanium-based precursors. Then they removed the OSDA, leaving behind a germanosilicate chiral framework (shown).
Confirming that each OSDA enantiomer indeed resulted in a molecular sieve of the predicted chirality required developing a novel electron crystallography technique. That aspect of the work, which was described in a paper published at the same time as the PNAS study, was led by Osamu Terasaki, an electron microscopist who holds positions at ShanghaiTech University and Stockholm University. The technique involves recording a series of diffraction patterns from a single crystal in an electron microscope as the crystal is tilted about a characteristic crystallographic axis (Nat. Mater. 2017, DOI: 10.1038/nmat4890).
To demonstrate the chiral zeolites’ potential usefulness, the researchers used them to conduct catalytic epoxide ring-opening reactions. The R and S enantiomers of the molecular sieve yielded corresponding enantiospecific diol products.
Synthesizing enantiomerically pure or enriched zeolites is a key scientific issue that can have important industrial applications, says Avelino Corma, a leading zeolite specialist at Polytechnic University of Valencia. “The authors unambiguously prove they have synthesized each enantiomeric form of the zeolite,” he says.
Alexander Katz, a chirality specialist at the University of California, Berkeley, remarks that this is “a beautiful testament to the power of rational functional-materials design.” This type of chiral shape selectivity can, in principle, be combined with various forms of chirality to cause amplification in enantioselectivity of other adsorption and catalytic processes, he adds


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter